Figure 6.
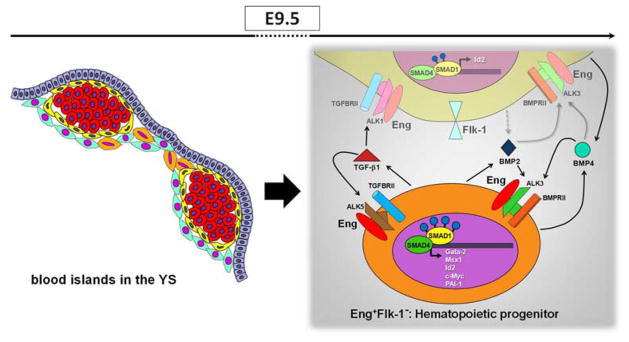
A model to illustrate how Eng and TGFβ1/BMP2/BMP4 may mediate induction of yolk sac hematopoiesis. Schematic representation (left panel) shows the localization of Eng+ cells in E9.5 blood islands. E+F+ and E+F− cells are represented in yellow and orange, respectively. Red round cells represent primitive erythrocytes. E+F+ cells represent endothelial cells whereas E+F− cells represent hematopoietic progenitors. The later secret high levels of TGFβ1 (right panel, represented in orange), which may signal in both hematopoietic (E+F−) and endothelial (E+F+) cells since both fractions contain the TGFβ receptor complex. In E+F− cells, TGFβ1 may bind to ALK5/TGFβRII/Eng, which results in high levels of PAI-1, a process mediated by SMAD2/3 (not shown). Nevertheless, the highest levels of pSMAD1/5 are observed within this fraction, and may result from BMP2/4 signaling, as E+F− cells display ALK3/BMPRII receptors, and high levels of corresponding target genes, including c-Myc, Gata2, Msx1 and Id2. More important, all target genes are drastically reduce in YS from Eng−/− embryos. E+F+ cells also exhibit significant phosphorylation of SMAD1/5, which may result from TGFβ1/ALK1 and BMP2/4 signaling. The important role played by endoglin in the YS, is possibly the modulation of the activation of TGFβ1 and BMP2/4 signaling pathways, and consequently the activation of important genes involved in the hematopoiesis. Grey dashed arrows represent possible secreted molecules, and grey solid arrows represent potential signaling pathways.
