Abstract
Cells in vivo are exposed to a complex signaling environment. Biochemical signaling modalities, such as secreted proteins, specific extracellular matrix domains and ion fluxes certainly compose an important set of regulatory signals to cells. However, these signals are not exerted in isolation, but rather in concert with biophysical cues of the surrounding tissue, such as stiffness and topography. In this review, we attempt to highlight the biophysical attributes of ocular tissues and their influence on cellular behavior. Additionally, we introduce the proteins YAP and TAZ as targets of biophysical and biochemical signaling and important agonists and antagonists of numerous signaling pathways, including TGFβ and Wnt. We frame the discussion around this extensive signaling crosstalk, which allows YAP and TAZ to act as orchestrating molecules, capable of integrating biophysical and biochemical cues into a broad cellular response. Finally, while we draw on research from various fields to provide a full picture of YAP and TAZ, we attempt to highlight the intersections with vision science and the exciting work that has already been performed.
1. Introduction
Researchers have long recognized the role of biochemical signaling in cell behavior. The presence of soluble factors, specific extracellular matrix (ECM) components, and ions are known to play key roles in signaling within and among cells. However, there is a growing recognition that biophysical attributes of the cellular microenvironment play an equally important role in the signaling milieu. Of special interest are the topographic features and stiffness of the cells’ microenvironment. As described in numerous reviews, the in vivo cellular microenvironment is composed of a complex network of ECM proteins, resulting in a soft, topographically featured substrate far different than the stiff, flat surfaces of tissue culture plastic typically used for in vitro studies (Guilak et al., 2009; Li et al., 2005; Lu et al., 2012; von der Mark et al., 2010). The response to biophysical stimuli, commonly referred to as mechanotransduction, has been linked to multiple changes in cell behaviors such as stem cell differentiation, metastatic potential of cancer cells and phenotypic changes in somatic cells. The presentation of surfaces possessing biomimetic biophysical attributes also alters cellular response to soluble signaling molecules, surface chemical cues, therapeutic agents and other biophysical cues.
This is certainly no less true in the specialized tissues of the eye. Optical clarity depends on the highly ordered and relatively stiff collagen networks of the corneal stroma, while only a small distance away corneal epithelial and endothelial cells rest on far softer basement membranes. In the back of the eye, the optic nerve is supported by the organized fibrous network of the lamina cribrosa and retinal pigmented epithelium rests on Bruch’s membrane. Intraocular pressure is regulated in part by outflow through the trabecular meshwork. Importantly, changes in the topography or stiffness of these structures have been linked to cellular dysfunction and disease progression. Understanding mechanotransduction is therefore a central question in the vision sciences. While there remains a paucity of studies about this in ocular biology, there is a rich history of mechanotransduction research in other systems that can be drawn upon when considering the importance and potential molecular mechanisms that participate in mechanotransduction in ocular structures.
Early research on mechanotransduction focused on ECM binding domains and adhesion complex proteins that were involved in cellular linkage to the extracellular environment. Later, the importance of the cytoskeleton and its linkage to the nucleus was investigated. However, a knowledge gap remained in the translation of the mechanical signals into observed transcriptional changes. A recent paper by Dupont and colleagues identified two transcriptional co-activators Yes-associated protein (YAP) and transcriptional co-activator with PDZ-binding motif (TAZ) as necessary for the transcriptional and phenotypic changes associated with alterations in the biophysical attributes of the cellular microenvironment (Dupont et al., 2011). YAP and TAZ were initially studied in their role as the primary effectors of the Hippo tumor suppression pathway but a growing body of literature suggests a much more complex picture of their functionality. Situated at the center of at least four signaling pathways (biophysical, Hippo, TGFβ/BMP, Wnt) and influencing several more (Retinoblastoma, IGF, PI(3)K/Akt), YAP and TAZ are positioned to serve as orchestrating molecules, integrating biophysical cueing into multiple potent signaling cascades (Figure 1). While we will discuss the many functions of YAP/TAZ that have been found in other systems, their function is known to be highly context dependent. This necessitates both tissue specific investigations and understanding of the cellular microenvironment. Therefore, we begin this review by describing ocular mechanobiology, highlighting known changes with disease.
Figure 1. YAP/TAZ Influences Multiple Signaling Pathways.
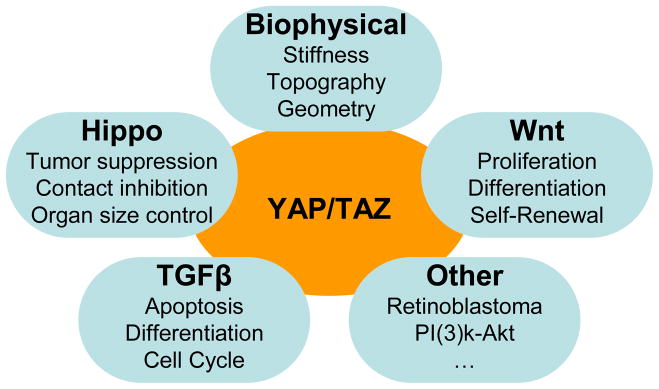
YAP/TAZ are best known as the primary targets of Hippo signaling and have recently been implicated in biophysical signaling. Additionally, YAP/TAZ expression, phosphorylation, and localization regulate TGFβ, Wnt, and a host of other proliferation and differentiation control pathways. Note: This schematic is simplified to clarify the major interactions in YAP/TAZ signaling, and is not intended to exhaustively represent all known YAP/TAZ interactions.
2. Mechanobiology of the Eye
The soft and textured tissues of the eye’s extracellular matrix could not be more different from the typical in vitro cultureware. In addition, experiments utilizing cultureware are unable to interrogate the effects of changing tissue mechanical properties in disease processes. In most cases it is unclear whether changing topography or the stiffness is an early cause of the disease, a mechanism of progression, or a late symptom of the disease process. What is known is that ocular pathologies are rife with examples of changing biomechanics. Below, we discuss a few key examples of ocular disease where mechanics appears to either contribute to or result from the pathology.
2.1 Mechanical properties in ocular tissues and pathology
Recent studies have reported the stiffness profile for the distinct components of the human cornea (Last et al., 2009; Last et al., 2012) as well as the detailed organization of the stromal collagen network (Winkler et al., 2011). These reports provide a critical backdrop for the interpretation of corneal pathologic states such as keratoconus. Keratoconus is a disease characterized (and named) by conical geometry of the cornea, brought about by thinning of the stroma (Rabinowitz, 1998). While the etiology of the disease is poorly understood, a key component is changes the biomechanical properties of the cornea (Ortiz et al., 2007; Shah et al., 2007; Wolffsohn et al., 2012). As a potential cause, the stroma is believed to be damaged by increased proteolysis and decreased protease inhibitor activity (Sawaguchi et al., 1994; Sawaguchi et al., 1989; Zhou et al., 1998). Importantly, while the biochemical makeup of the stroma appears not to be altered, there are changes to the structure and ultrastructure of the stroma (Daxer and Fratzl, 1997; Meek et al., 2005; Patey et al., 1984; Zimmermann et al., 1988). Additionally, the disease can be alleviated through UV-riboflavin collagen crosslinking (Goldich et al., 2012). Together, these data suggest that while the biochemical makeup of the stroma may be identical, decreased crosslinking may weaken the stroma, leading to degeneration of the cornea.
Age has been implicated in loss of accommodation (presbyopia), although the mechanism remains incompletely understood (McGinty and Truscott, 2006). As accommodation requires deformation of the lens by the ciliary muscle, lens stiffness has been long suspected as a likely cause (Fisher, 1971). Recent research has confirmed increasing stiffness with age and additionally describing variations in stiffness between the nucleus and the cortex (Fisher, 1971; Hollman et al., 2007; Weeber et al., 2007; Wilde et al., 2012). Additionally, this increase in stiffness correlates well with a known decrease in the transport of important small molecules to the nucleus, which is implicated in age related nuclear cataract formation (Moffat et al., 1999; Sweeney and Truscott, 1998). It was recently been proposed that changes in biomechanics of the lens are related to decreased transport and the resulting cataract formation (McGinty and Truscott, 2006).
The mechanics of the retina have also recently been investigated. A report by Davis and colleagues found that Mueller cells have a gene expression profile which is dependent on substratum stiffness (Davis et al., 2012). Additionally, CTGF was upregulated on soft hydrogels in comparison to glass. CTGF is a potent regulator of ECM structure, and is implicated in the progression of age-related macular degeneration (Nagai et al., 2009). There is also evidence of altered biomechanics in the retina with age. Bruch’s membrane, the border between the retina and choroid, has long been known to stiffen with age (Fisher, 1987). Beyond the changes in stiffness, it also roughly doubles in thickness and undergoes biochemical and ultrastructural changes (Ramrattan et al., 1994; Zarbin, 2004). These changes are suspected to contribute to age-related macular degeneration, in addition to damage of Bruch’s membrane and a number of ECM and protease modifications (Spraul et al., 1999; Zarbin, 2004).
Cupping of the optic nerve head and changes to the optic disk are considered hallmarks for the onset and progression of glaucoma, and can even precede loss in the visual field (Quigley, 1993). The mechanics of the lamina cribrosa (LC) and peripapillary sclera (PS) have been suspected to play a role in the susceptibility of glaucomatous damage (Sigal and Ethier, 2009). This is supported by a recent study correlating scleral biomechanics of different mouse strains with rate of retinal ganglion cell loss in experimental glaucoma (Nguyen et al., 2013). In humans, the biochemical makeup of the LC is also known to change with age, and it is suspected this leads to increased stiffness and decreased resilience (Albon et al., 1995; Albon et al., 2000; Morrison et al., 1989). Additionally, elevated intraocular pressure, a common indicator of glaucoma, also acutely increased LC/PS stiffness (Thornton et al., 2009). A diabetic rat model exhibited elevated LC and PS stiffness, which was proposed as a mechanism for linking diabetes and glaucoma, although that link is unclear (Dielemans et al., 1996; Ellis et al., 2000; Klein et al., 1994; Terai et al., 2012). The above studies all suggested an increase in stiffness with glaucoma, however, a recent study reported contrasting results, showing a decrease in LC and PS stiffness with pseudoexfoliation glaucoma (Braunsmann et al., 2012).
In humans, glaucoma is also known to involve changes in the anterior segment through the regulation of aqueous humor outflow through the human trabecular meshwork (HTM) and Schlemm’s canal (SC). The HTM is a complex, three-dimensional structure comprised of trabecular meshwork cells and associated ECM consisting of interwoven collagen beams and perforated sheets (Acott and Kelley, 2008; Johnson, 2006). HTM cells, depending on the region of the HTM, either form sheets covering ECM structures or are scattered throughout the ECM forming occasional gap and adherens junctions (Bhatt et al., 1995; Gong et al., 2002; Grierson et al., 1978; Inomata et al., 1972). The ultrastructure of the HTM is known to change in glaucoma (Lutjen-Drecoll, 2005; Rohen et al., 1993). These structural changes correlate with progression of nerve damage, further implicating the importance of the HTM in disease progression (Gottanka et al., 1997). Adjacent to the HTM, the endothelium of SC has additionally been identified as a potential regulator of outflow through the formation of intra- and inter-cellular pores (Allingham et al., 1992; Johnson et al., 2002).
Recent reports have begun to interrogate the mechanics of the HTM and SC in the context of glaucoma. These have demonstrated that cytoskeletal disruption through pharmacological agents investigated as potential glaucoma therapeutics, decreases the intrinsic stiffness of SC and HTM cells (McKee et al., 2011c; Zhou et al., 2012). Last and colleagues used atomic force microscopy (AFM) to measure the stiffness of the juxtacanilicular region of the HTM (JCT) of normal and glaucomatous donor tissue (Last et al., 2011). These exciting results revealed a dramatic increase in the elastic modulus of the meshwork, from 4.0 ± 2.2 kPa in normal HTM to 80.8 ± 32.5 kPa in glaucomatous HTM. A seemingly conflicting report by Camras and colleagues related tensile tests of dissected HTM directly to outflow facility from perfusion tests (Camras et al., 2012). They reported that normal HTM elastic modulus (515 ± 136 kPa) varied with outflow facility, exactly the opposite of what would be expected from Last’s correlation between stiffness and glaucoma. However, these experiments have key differences which likely account for the different conclusions. Last et al used AFM, which measures local tissue properties at the nano to micro scales, while Camras and coworkers measured the deformation of large HTM segments in tension, effectively measuring a circumferential, or “hoop”, modulus. The difference in measurement can clearly be seen in different values each measurement provides (4.0 ± 2.2 kPa with AFM to 515 ± 136 kPa with tensile testing). Another important limitation of the above studies is that they were performed on excised tissue. This results in a loss of both the influence of surrounding tissue and the soluble signaling milieu provided by the aqueous humor. Both these factors can influence the external and intrinsic contraction of the tissue, influencing mechanics. Mouse models with increased contractility of TM cells or adjacent ciliary muscle cells exhibit increased and decreased flow resistance, respectively (Inoue-Mochita et al., 2009; Junglas et al., 2012). These results suggest increased tension within or around TM and SC change the overall tissue structure and stiffness and provide a potential active mechanism for modulating outflow.
2.2 Measurement of tissue mechanics
As implied above in the discussion of HTM mechanics, measurements of tissue properties are complex and researchers typically use simple models to generalize the results of specific mechanical tests such as AFM or tensile testing. In order to reduce the results to single elastic modulus values from complex force/deformation plots, assumptions that tissue is homogeneous, elastic and isotropic are often employed. These are common assumptions when studying tissue mechanics (reviewed in the context of soft tissue here (McKee et al., 2011a)), but one, isotropy, deserves special mention. In the HTM mechanics described above, we can clearly see that the microscale indentation modulus and the hoop modulus are dramatically different, showing that the HTM is anisotropic. This is likely due to the circumferential fiber alignment (Camras et al., 2012) and large open spaces in the JCT (Fuchshofer et al., 2006), providing substantial resistance along the fibers but allowing relatively little for perpendicular deformations, such as measured with Last’s experiments. Discrepancies such as these serve as a reminder to carefully consider the assumptions and limitations built into all experimental techniques during the interpretation of results.
2.3 Cellular biomechanics and behavior
Do these biomechanical changes influence cell behavior? Numerous studies from our laboratory and others have shown that the physiologically-relevant biophysical attributes of substratum stiffness and substratum nanotopography impact cytoskeletal organization (Davis et al., 2012; Dunn and Brown, 1986; Karuri et al., 2006; Liliensiek et al., 2010; McKee et al., 2011b; McKee et al., 2011c; Morgan et al., 2012; Oakley and Brunette, 1993; Raghunathan et al., 2013a; Uttayarat et al., 2008; Wood et al., 2011a; Wood et al., 2011b). Despite this, most in vitro experiments are conducted on tissue culture plastic or glass with stiffness well outside the physiological range (GPa vs. kPa) and lack topographic features, resulting in profoundly altered cytoskeletal phenotypes. But the impact of biophysical cues goes beyond cytoskeletal regulation and has far-reaching effects on all aspects of cellular behavior including response to soluble cytoactive factors and therapeutic agents.
As mentioned above, substratum stiffness influences Mueller cell proliferation, structure, and gene expression (Davis et al., 2012). Similarly, substratum topography influences the structure and gene expression of corneal epithelial cells (Dreier et al., 2012). Several recent in vitro studies have documented HTM cells alter their phenotype, gene/protein expression, and response to soluble cytoactive factors (such as latrunculin B and TGFβ) in response to the presentation of biomimetic surface cues (Han et al., 2011; McKee et al., 2011c; Raghunathan et al., 2013b; Schlunck et al., 2008; Thomasy et al., 2012; Wood et al., 2011a). Despite the consensus that substratum stiffness profoundly influences cell behavior, there are inconsistencies in some of the data reported. For example, myocilin, a protein long associated with glaucoma, is reported as both positively (Raghunathan et al., 2013b; Thomasy et al., 2012) and negatively (Schlunck et al., 2008) regulated by increasing hydrogel stiffness, although there is agreement that biomimetic stiffness upregulate myocilin when compared to tissue culture surfaces. Conflicting reports such as these emphasize that differences in the details of experimental design, such as specifics of substrate preparation, extracellular matrix coating, time in culture, and other variables likely modulate cellular responses to substratum stiffness.
3. YAP/TAZ as orchestrating molecules
While the above studies demonstrate the importance of mechanotransduction in ocular biology and pathology, we are left with the open question of the molecular effectors and pathways that interpret biophysical cues and transduce them to determine cellular behaviors. A full discussion of cellular mechanotransduction is beyond the scope of this or any single review and we will instead focus on the unique role of YAP/TAZ. The majority of studies involving YAP/TAZ were initially conducted and discussed in the context of Hippo signaling, necessitating a firm understanding of the Hippo pathway before progressing to exploring YAP/TAZ as relays of biophysical cues and their extensive crosstalk with other signaling pathways.
3.1 YAP/TAZ as Hippo pathway targets
The Hippo pathway centers on a cascade of kinase activations which ultimately inhibit an anti-apoptotic/pro-proliferative gene program. A highly conserved pathway, Hippo plays a central role in reaching and maintaining appropriate organ size (Dong et al., 2007). Precise control of this pathway is essential for proper development and stem cell regulation (Barry and Camargo, 2013; Mauviel et al., 2012). This has been shown quite dramatically in Drosophila, where mutations in the Hippo pathway typically lead to tissue overgrowth. For this reason, Hippo has been extensively studied in the context of tumorigenesis and metastasis. Hippo dysregulation has been identified as a frequent component of cancer (Harvey et al., 2013). While much is still unknown about how cells sense their environment, Hippo clearly plays an important role.
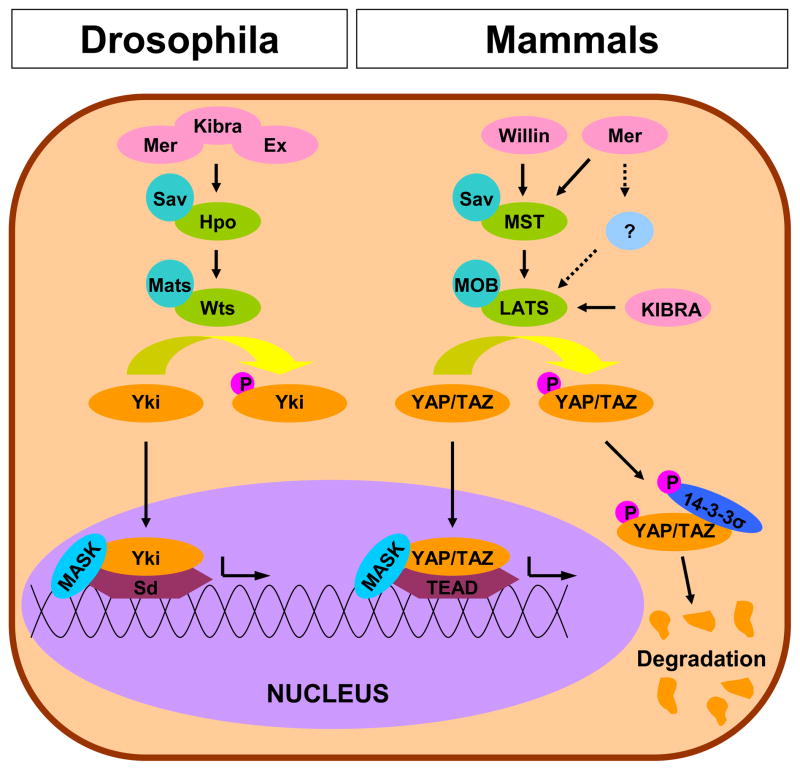
In an effort to be succinct, we will discuss key points of the pathway and direct interested readers to other recent reviews of the subject (Zhao et al., 2010a; Zhao et al., 2010b). A highly conserved pathway, Hippo exhibits both astonishing complexity and profound elegance (Figure 2). We have attempted to highlight key aspects of the pathway so that the reader can gain a fundamental understanding, however, even this simplified description will probably require the reader to refer to the schematic in Figure 2 repeatedly. The core components of the Hippo pathway were originally described in Drosophila (Figure 2; left side) and compose a linear pathway of kinases that act to repress the nuclear translocation of the transcriptional coactivator Yorkie (Zhao et al., 2010a; Zhao et al., 2010b). When activated, Hippo (Hpo) and Salvador (Sav) complex together and initiate the Hippo cascade (Harvey et al., 2003; Jia et al., 2003; Kango-Singh et al., 2002; Pantalacci et al., 2003; Tapon et al., 2002; Udan et al., 2003; Wu et al., 2003). Hpo/Sav phosphorylates a complex of Warts (Wts) and mob as tumor suppressor (Mats) (Justice et al., 1995; Lai et al., 2005; Wei et al., 2007; Xu et al., 1995). Activation of the Wts/Mats complex deactivates Yorkie (Huang et al., 2005) (Yki) by phosphorylation. This triggers cytoplasmic retention and inhibits the formation of a complex involving Multiple Ankyrin-repeat Single KH (MASK) proteins and the anti-apoptotic and pro-proliferative transcription factor Scalloped (Sd) (Goulev et al., 2008; Sansores-Garcia et al., 2013; Sidor et al., 2013; Wu et al., 2008; Zhang et al., 2008b). This Hpo/Sav-Wts/Mats mediated downregulation of Sd transcription is the primary mechanism of tumor suppression and growth control activity of the Hippo pathway (Zhang et al., 2008b). The core components and sequence of the pathway are heavily conserved in mammals and are depicted in a simplified manner on the right side of Figure 2 (Dong et al., 2007; Zhang et al., 2008a).
Figure 2. The Hippo Pathway in Drosophila and Mammals.
Canonical Hippo pathway complexes act to phosphorylate Yki or YAP/TAZ and prevent assembly of the Sd or TEAD transcriptional complexes with MASK. Phosphorylated YAP/TAZ is cytoplasmically retained by 14-3-3σ and targeted for degradation. Similar coloration is used to highlight the level of homology and conservation of the pathway between Drosophila and mammals. Note: This schematic is simplified to clarify the major components in Hippo pathway.
Homologous to Hpo/Sav, the Mammalian Sterile Twenty kinases Mst1 and Mst2 (Mst) complex with Salvador 1 (Sav1) upon activation of the Hippo pathway (Callus et al., 2006). This leads to the phosphorylation and activation of a complex of large tumor suppressor (Lats) 1/2 and Mps one binder 1A and 1B (MOB), homologous to Wts/Mats in Drosphila (Chan et al., 2005; Chow et al., 2010; Hao et al., 2008; Hergovich et al., 2005; Hergovich et al., 2006; Zhang et al., 2008a). After the formation of the Lats/MOB complex there is an important difference in mammalian pathway because there are two Yki homolouges, YAP and TAZ (Hao et al., 2008; Lei et al., 2008; Liu et al., 2010a; Zhang et al., 2008a; Zhao et al., 2007). Similar to Drosophila, phosphorylation of YAP and TAZ triggers cytoplasmic retention and prevents interaction with the mammalian Sd homologues, TEA domain (TEAD) family members TEAD 1–4 (Liu et al., 2010b; Mahoney et al., 2005; Ota and Sasaki, 2008; Zhang et al., 2009; Zhao et al., 2008). Similar to Drosophila, these YAP/TAZ/TEAD complexes are dependent on the presence of MASK for full activity (Sansores-Garcia et al., 2013). The final result, suppressing the anti-apoptotic and pro-proliferation TEAD, is homologous to the function of Drosophila Hippo. It is important to note that while TEAD is the most frequently cited transcription factor partner to YAP/TAZ, other transcription factors have been shown to play a role as well (e.g. RUNX, ErbB-4, p73, TTF-1, PPARγ) (Basu et al., 2003; Hong et al., 2005; Komuro et al., 2003; Omerovic et al., 2004; Park et al., 2004; Strano et al., 2001; Yagi et al., 1999; Zaidi et al., 2004).
The mechanism of cytoplasmic retention is as important as the kinase activity described above, and deserves further explanation. As described above, activation of the Hippo pathway leads to the phosphorylation of YAP and TAZ, allowing them to be cytoplasmically sequestered by the scaffolding protein 14-3-3σ and ultimately targeted for destruction (Huang et al., 2012; Kanai et al., 2000; Lei et al., 2008; Liu et al., 2010a; Zhao et al., 2010c; Zhao et al., 2007). Both YAP and TAZ have multiple phosphorylation sites, although serine-127 (S127) on YAP and serine-89 (S89) on TAZ are the most frequently discussed Lats targets. Importantly, the high sequence homology between these sites can cause antibody crossreactivity (Lei et al., 2008).
While the core of the Hippo pathway has been well described, less is known about upstream activators, although many have been implicated (Boggiano and Fehon, 2012; Grusche et al., 2010). The full list of these upstream components is outside the scope of this review but three proteins deserve mention as the canonical activators. In Drosophila, Merlin (Mer), Expanded (Ex), and Kibra complex and directly bind and activate Hpo/Sav (Genevet et al., 2010; Hamaratoglu et al., 2006; Yu et al., 2010). Importantly, these proteins act synergistically and redundantly to activate Hippo, as none are independently required for Hippo activation. Single mutants do exhibit decreased Hippo activation, however, the phenotype is enhanced in double mutants (Hamaratoglu et al., 2006; Yu et al., 2010). Consistent with Drosophila, the human orthologs, Mer/NF2, Willin/FRMD6, and KIBRA/WWC1, have been individually identified as activators of the Hippo pathway (Angus et al., 2012; Moleirinho et al., 2012; Xiao et al., 2011; Yu et al., 2010; Zhang et al., 2010). The parallelism observed in Drosophila is mirrored in mammalian cells. KIBRA appears to activate Lats in an Mst-independent fashion, while Willin appears to act through Mst and Merlin acts through both Mst dependent and independent mechanisms (Angus et al., 2012; Genevet et al., 2010; Kim et al., 2011; Murray et al., 2012; Xiao et al., 2011). The dispensability of Mst in downstream Hippo activation is consistent with other observations, but should not be construed as a lack of importance, as Mst deficiency leads to severe in vivo phenotypes. Independently, Mst1 and Mst2 deficiency doesn’t alter viability but can lead to increases in tumor formation and, in the case of Mst1, a loss of naïve T cells (Zhou et al., 2009; Zhou et al., 2008). Double knockouts are embryonic lethal with conditional double knockouts in the liver or intestines result in dysplasia and tumor formation (Song et al., 2010; Zhou et al., 2009; Zhou et al., 2011). Consistent with the level of complexity described above, the subset of Hippo target genes activated through Mer, Willin, or Kibra are distinct (Moleirinho et al., 2012). As a final note, several of the upstream regulators have been identified as Hippo targets, revealing a negative feedback mechanism which may help maintain Hippo at a steady state (Genevet et al., 2010; Hamaratoglu et al., 2006; Xiao et al., 2011).
Relevant to the subject of this review, canonical Hippo signaling has already been identified in the eye. Recent reports have linked deficient YAP/TAZ-TEAD1 binding to the pathogenesis of Sveinsson’s chorioretinal atrophy, an autosomal dominant disease (Fossdal et al., 2004; Kitagawa, 2007). Mer deficiency led to cataract formation in mice, and this was partially rescued by Yap null heterozygosity (Zhang et al., 2010). Additionally, Hippo target genes include numerous genes implicated in a variety of ocular pathologies including matrix metalloproteinases 7/12, interleukin 1β, transforming growth factor (TGF) β2, TGFβ receptor 1, connective tissue growth factor (CTGF), serpine-1, transglutaminase 2, and type IV collagen α3 (Dong et al., 2007; Dupont et al., 2011; Ota and Sasaki, 2008; Zhang et al., 2009; Zhao et al., 2008).
3.2 YAP/TAZ and contact inhibition
Building upon their role as pro-proliferative proteins, much research has been done exploring YAP/TAZ deactivation in contact inhibition. Epithelial-mesenchymal transition (EMT), characterized in part by the loss of contact inhibition, contributes to multiple ocular pathologies including dysregulated corneal wound healing, cataracts, and proliferative vitreoretinopathy after retinal detachment (Aomatsu et al., 2012; de Iongh et al., 2005; Kawashima et al., 2010; Liu et al., 2010b; Martinez and de Iongh, 2010). YAP/TAZ have been linked to EMT both in general and in the eye specifically (Lei et al., 2008; Liu et al., 2010b; Wang et al., 2011; Zhang et al., 2009). Perhaps the strongest evidence for involvement of YAP/TAZ in contact inhibition is the repeated observation of their nuclear exclusion in high density cultures (Kim et al., 2011; Ota and Sasaki, 2008; Silvis et al., 2011; Varelas et al., 2010b; Wang et al., 2012; Zhao et al., 2011; Zhao et al., 2007). This result is not limited to in vitro findings, with YAP and TAZ exhibiting cell contact-dependant localization in early development of mouse embryos (Nishioka et al., 2009; Varelas et al., 2010b). In the inner cell mass of the embryo, where cells are contacted on all sides, YAP and TAZ are excluded from the nucleus, while surface cells exhibit nuclear localization.
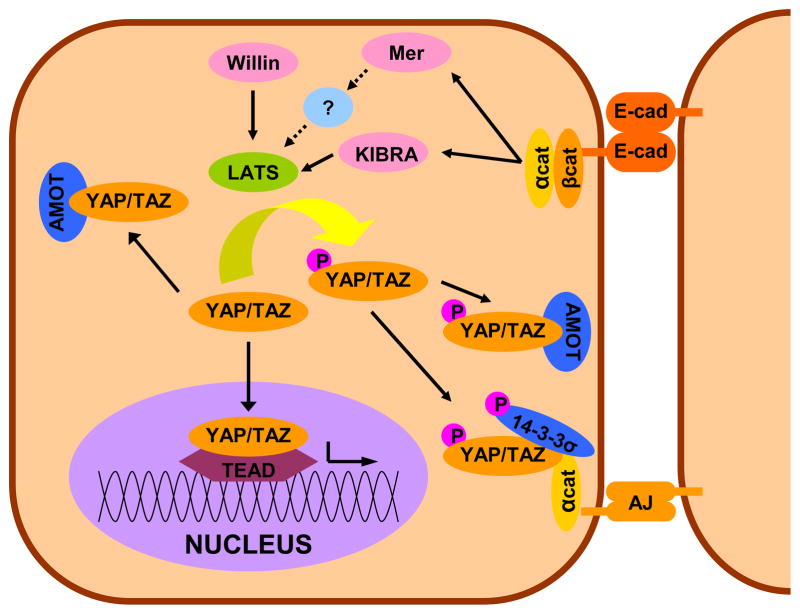
The obvious candidate for regulating YAP/TAZ in response to cell-cell contact is the Hippo pathway, which indeed plays a role. However, cell-cell contact regulates YAP/TAZ through multiple pathways, three of which are described below. E-cadherin, a major component of cell-cell junctions, is known to sequester YAP/TAZ to the cytoplasm and this activity is central to E-cadherin’s role as a tumor suppressor (Nishioka et al., 2009). Further work emphasized that the E-cadherin mediated signaling occurs through both Hippo and yet to be identified parallel kinase pathways which ultimately phosphorylate YAP/TAZ, allowing for their sequestration (Kim et al., 2011). Angiomoitin (AMOT) and its paralogs (AMOT like 1/2) have also been shown to sequester YAP/TAZ in the cytoplasm (Chan et al., 2011; Oka et al., 2012; Varelas et al., 2010b; Wang et al., 2011; Zhao et al., 2011). The AMOTs can sequester YAP/TAZ through direct protein-protein interactions regardless of the phosphorylation status of YAP/TAZ (Chan et al., 2011; Wang et al., 2011; Zhao et al., 2011). The third potentially parallel system mediated by α-catenin has been identified by two studies in skin (Schlegelmilch et al., 2011; Silvis et al., 2011). While both studies show independence from the core Hippo machinery and E-cadherin, they differ in the specific mechanism. Schlegelmilch and colleagues reported that α-catenin forms a complex with 14-3-3σ that both sequesters YAP and inhibits dephosphorylation by a promiscuous phosphatase PPA2. Silvis and colleagues provide evidence for a phosphorylation-independent direct sequestration of YAP by α-catenin. A simplified view of these multiple contact inhibition modalities is presented in Figure 3.
Figure 3. Cell-cell Contact and YAP/TAZ.
Cell-cell contact can inhibit YAP/TAZ transcriptional activity both by sequestration to adherins junctions (AJ) in an α-catenin dependent fashion and through E-cadherin/catentin dependant activation of the Hippo pathway. Additionally, AMOT can sequester YAP/TAZ regardless of phosphorylation status. Note: This schematic is simplified to highlight the major factors in cell contact inhibition of YAP/TAZ.
3.3 YAP/TAZ as relays of mechanical signaling
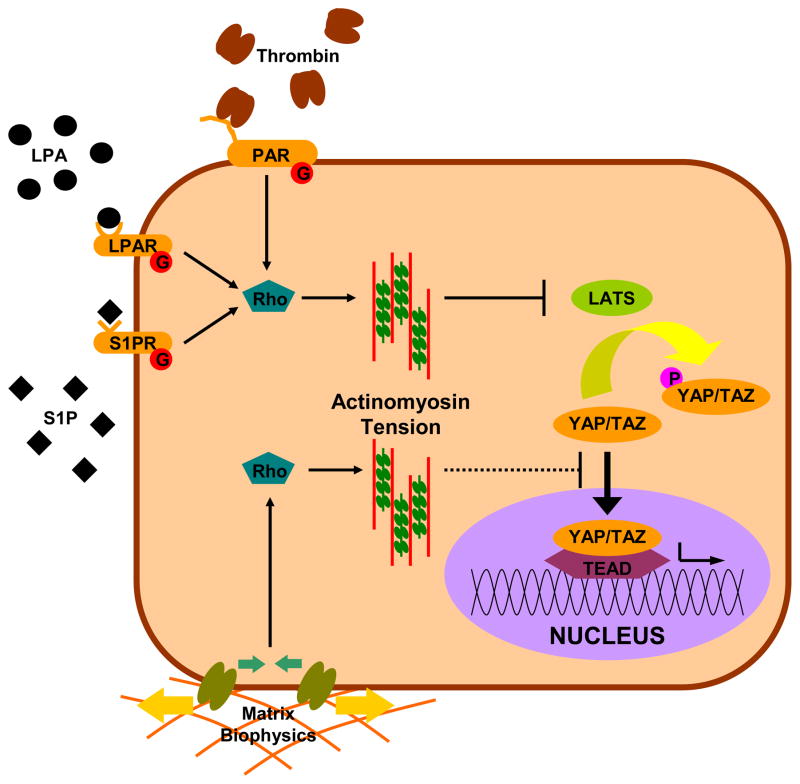
With this foundation in Hippo signaling, we turn to the role of YAP/TAZ in a central theme of this review: mechanotransduction. As the reader will quickly become aware, it is difficult to separate out the influence the biophysical attributes of the microenvironment of the cell from the host of factors that can influence cytoskeletal mechanics such as G-protein coupled receptor (GPCR) signaling. As such, we will discuss them together and refer the reader to Figure 4 for clarification. The initial discovery of YAP/TAZ as relays of mechanical signals is relatively recent (Dupont et al., 2011). In that report, the nuclear localization and transcriptional activity of YAP was inhibited by soft substrates, restrictive geometry, and actinomyosin disruption. This was independent of phosphorylation and the core Hippo components Mer and Lats. However, given the complexity and cell type specificty of YAP/TAZ regulation, it is not clear that mechanical signaling is uniformly independent of Hippo. Indeed, another report found geometry and actinomysin regulated YAP at or upstream of Lats activity (Wada et al., 2011). An additional report on the role of Hippo in anoikis (detachment induced apoptosis), confirms a role for Lats and actin in mechanical signaling, but didn’t find a role for cytoskeletal tension (Zhao et al., 2012). In this study, ROCK and myosin inhibition do not recapitulate the results of filamentous actin inhibition. Disagreement at the point at which mechanical signaling links to Hippo is good example of the context-sensitive nature of YAP/TAZ, and similar discrepancies are detailed throughout this review. More important is where these studies agree: mechanical cues such as geometry and stiffness are crucial regulators of YAP/TAZ and this regulation seems to take place through regulation of the actinomyosin cytoskeletal system. The concept of Hippo being linked to the actin cytoskeleton at or above Lats is supported by the recent research in Drosophila, where studies indicated increasing or decreasing filamentous actin resulting in increased or decreased Yki activity, respectively, and this involved the Lats homologue, Wts (Rauskolb et al., 2011; Sansores-Garcia et al., 2011). Actinomyosin can also be modulated by more traditional biochemical cues, and these have also been shown to regulate YAP. Growth factor like lipids such as lysophosphatidic acid (LPA) and sphingosine-1-phosphate (S1P) and proteases such as thrombin have recently been identified as potent activators of YAP, acting through GPCR signaling and the actinomyosin cytoskeleton (Miller et al., 2012; Mo et al., 2012; Yu et al., 2012). Importantly, two of three studies (Mo et al., 2012; Yu et al., 2012) indicate a role for Lats in transducing GPCR signals to YAP, while the third didn’t investigate that question (Miller et al., 2012). A key consequence of the above findings is that by regulating the cytoskeleton, biophysical cues and Rho-signaling can regulate the localization and phosphorylation of YAP/TAZ.
Figure 4. Mechanical regulation of the Hippo pathway.
Hippo is regulated by multiple signals generated by the physical (matrix stiffness) and biochemical (LPA, S1P, thrombin) environment. Importantly, many of these signals are modulated by tension in the actinomyosin cytoskeleton. Modulation of cytoskeletal mechanics through G-protein coupled receptors and matrix biophysics can likewise inhibit YAP/TAZ directly at the nuclear translocation stage or through activation of Hippo components. Note: This schematic is simplified to clarify the major components in the mechanical regulation of YAP/TAZ signaling.
The importance of cytoactive lipids such as LPA and S1P is also especially relevant to the eye. Two independent reports have recently identified autotaxin (the enzyme that produces LPA) as highly expressed and active in the aqueous humor (Iyer et al., 2012; Tokumura et al., 2012). Iyer and colleagues further showed that inhibiting autotaxin function (in fact reducing LPA) decreases intraocular pressure (IOP) in rabbits. This is consistent with other reports which showed decreased outflow facility with S1P and increased outflow facility with S1P receptor inhibition in enucleated mouse and human eyes (Boussommier-Calleja et al., 2012; Stamer et al., 2009; Sumida and Stamer, 2011). Tokumura and colleagues built upon previous studies revealing LPA and related phospholipids are potent mediators of corneal wound healing (Liliom et al., 1998; Watsky, 1995; Xu et al., 2007; Yin et al., 2008). Although none of these reports investigated the localization or activity of YAP/TAZ, the fact that the tissues of the anterior chamber are continuously exposed to factors known to activate YAP/TAZ certainly raises intriguing questions.
3.4 YAP/TAZ and TGFβ superfamily signaling
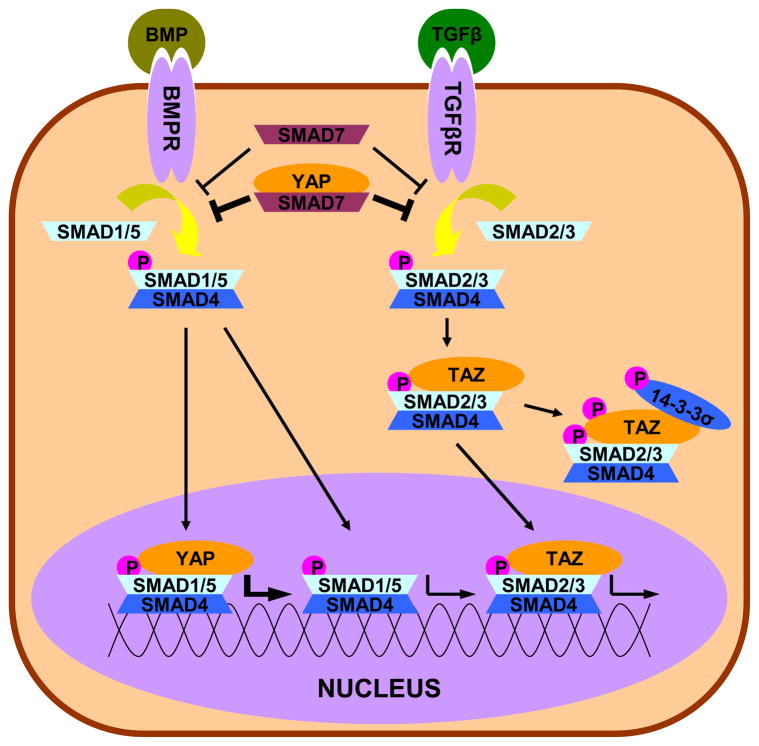
We’ve already identified three key regulators of YAP/TAZ localization and phosphorylation (Hippo, cell-cell contact, biophysical cues), and the YAP/TAZ story is still expanding. In addition to influencing TEAD transcription, YAP/TAZ localization regulates other signaling pathways well studied in the context of ocular health and disease, such as TGFβ (Figure 5). The TGFβ superfamily is a potent regulator of ECM turnover and composition and is implicated in numerous ocular disorders, including pathological fibrosis in corneal wound healing and cataract formation, as well the structural changes of both the TM and ONH present in glaucoma (Fuchshofer, 2011; Fuchshofer and Tamm, 2012; Jester et al., 1999; Saika, 2006; Saika et al., 2009; Tamm and Fuchshofer, 2007). A comprehensive presentation of TGFβ signaling is beyond the scope of this review and current reviews are available (Horbelt et al., 2012; Massague, 2012; Shi and Massague, 2003; Zi et al., 2012). Here, we summarize the core of the pathway. Cytokines of the TGFβ superfamily (most notably TGFβ1–3 and bone morphogenetic proteins (BMPs)) interact with membrane bound receptors that have serine/threonine kinase activity. The intracellular transducers of these receptors are the receptor SMADs (R-SMADs), notably SMAD1, 5, and 8 which are responsive to BMP signaling and SMAD2 and 3 which are responsive to TGFβ signaling. The R-SMADs complex with the co-mediator SMAD (Co-SMAD), SMAD4, can then shuttle to the nucleus where they act as transcription factors. This pathway can be blocked by inhibitory SMADs (I-SMADs), SMAD6 and 7. SMAD6 inhibits the phosphorylation of SMAD1, 5, and 8 and additionally inhibits the association of SMAD4 with SMAD1, both resulting in decreased BMP induced signaling. SMAD7 acts on all R-SMADs to inhibit their phosphorylation and also promote dephosphorylation, resulting in the inhibition of all TGFβ superfamily signaling. Importantly, SMAD6 and 7 are both targets of TGFβ superfamily signaling, providing a negative feedback mechanism within the pathway (Ishida et al., 2000; Nagarajan et al., 1999).
Figure 5. Crosstalk between YAP/TAZ and TGFβ.
TGF β superfamily signaling (especially TGFβ and BMP) is initiated by the binding of an extracellular ligand, which leads to the phosphorylation of the R-SMADs (SMADs 1/5 and 2/3 shown) and the formation of a complex with R-SMADs and a Co-SMAD (SMAD4). After translocation to the nucleus, these complexes initiate the TGFβ/BMP transcriptional program. Through direct interaction with TAZ, SMAD2/3 can be retained in either the cytoplasm or nucleus, depending on the localization of TAZ. Additionally, YAP can enhance the inhibitory effects of SMAD7 or enhance the transcription of SMAD1/5. Note: This schematic is simplified to clarify the major intersections of YAP/TAZ and TGFβ.
YAP/TAZ are known to interact in several ways with SMADs. The first report of such interaction was binding activity between YAP and the broadly inhibitory SMAD7, but not BMP specific inhibitory SMAD6. This interaction increased the localization of SMAD7 to active TGFβ receptors, resulting in elevated inhibition of R-SMAD phosphorylation (Ferrigno et al., 2002). To be clear, it is important to state that this report did not show that YAP is a requirement for SMAD7 function, but rather amplified it. In addition to its inhibitory functions via SMAD7, YAP also assists BMP specific signaling through a BMP dependent association with SMAD1/5. YAP co-precipitated with SMAD1/5 on the BMP target sites Id1 and Id2, and YAP depletion inhibited induction by BMP of the target genes Id1, Id2, and Id3 (Alarcon et al., 2009). TAZ has also been implicated in TGFβ signaling through a TGFβ-dependent association with SMAD2 and SMAD3 (Varelas et al., 2008; Varelas et al., 2010b). These reports identified TAZ (but not YAP) as required for SMAD2/3 nuclear accumulation and transcriptional activity in response to TGFβ signaling. Importantly, they also showed TAZ as a transcriptional target of TGFβ, identifying another feedback mechanism of the TGFβ pathway. Depending on the regulation of TAZ by other pathways, this could either result in positive or negative feedback, further emphasizing the role of YAP/TAZ as having influence in of diverse pathways.
3.5 YAP/TAZ and canonical Wnt signaling
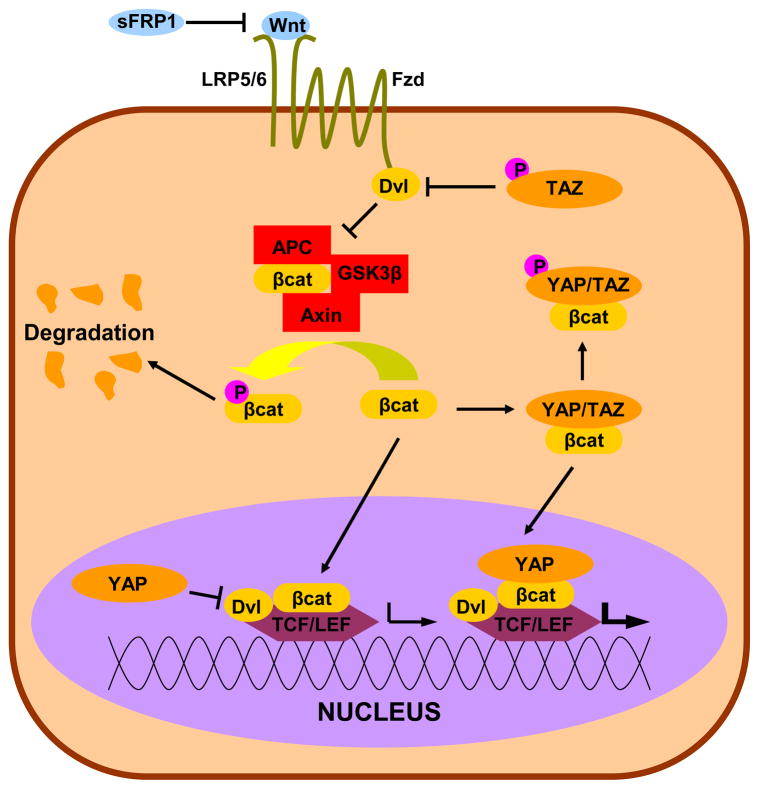
Similar to TGFβ, YAP/TAZ also have numerous points of intersection with canonical Wnt signaling (Figure 6), which has recently been implicated in glaucoma (Mao et al., 2012; Wang et al., 2008) and has long established roles in lens and retinal development, function and repair (de Iongh et al., 2006; Lad et al., 2009; Osakada et al., 2007). Additionally, Wnt is known to be a key pathway in regulating epithelial stem cell populations, and the limbal stem cell niche of the cornea is no exception (Blanpain et al., 2007; Kulkarni et al., 2010; Nakatsu et al., 2011). Again, we direct the reader to other comprehensive reviews on Wnt signaling (Clevers, 2006; Freese et al., 2010; Macdonald and He, 2012), but will attempt to summarize the pathway and association with YAP/TAZ below. The major effector of the Wnt pathway is β-catenin, with induces gene transcription through the T cell factor/lymphoid enhancer-binding factor (TCF/LEF) family of transcription factors. Typically, β-catenin is retained in the cytoplasm and targeted for degradation by a complex of Axin, adenomatous polyposis coli (APC), and glycogen synthase kinase 3β (GSK3β). The Wnts are secreted factors which bind to receptor complexes of Frizzled (Fzd) proteins and lipoprotein related proteins (LRP) 5/6, and this activity can be blocked by soluble factors such as soluble frizzled-related protein 1 (sFRP1). The Wnt-Fzd binding activity causes Dishevelled (Dvl) to inhibit the function of the Axin/APC/GSK3β complex, freeing β-catenin to translocate to the nucleus and act as a coactivator of transcription with TCF/LEF. Several studies have also pointed to a role of Dvl as a key component of the β-catenin/TCF/LEF complex (Gan et al., 2008; Itoh et al., 2005).
Figure 6. Crosstalk between YAP/TAZ and Wnt.
Canonical Wnt is initiated by the binding of a Wnt ligand to the Fzd/LRP receptor complex. This induces the inhibitory behavior of Dvl on the Axin/APC/GSK3β complex, freeing β-catenin to complex with Dvl and the transcription factor TCF/LEF and initiate the Wnt transcriptional program. YAP/TAZ can inhibit Wnt signaling through inhibition of Dvl in the cytoplasm (TAZ) or in the nucleus (YAP) or cytoplasmic sequestration of β-catenin (YAP). Alternatively, YAP can encourage the transcriptional activity of β-catenin. Note: This schematic is simplified to clarify the major intersections of YAP/TAZ and Wnt signaling.
Several known points of interaction between YAP/TAZ and Wnt signaling have been uncovered in the past few years and are show in Figure 6. Varelas and colleagues reported TAZ functioned as cytoplasmic antagonist of the Wnt pathway through Dvl, leading to a polycystic kidney phenotype in vivo (Varelas et al., 2010a). A recent report by Barry and colleagues reported a nuclear interaction between YAP and Dvl, resulting in suppression of Wnt mediated hyperplasticity in vivo and in vitro (Barry et al., 2012). Similarly, YAP and TAZ antagonize Wnt signaling through direct interaction with β-catenin (Imajo et al., 2012). Importantly, the study by Imajo and colleagues showed that the Wnt-suppressive activity of YAP was dependant on phosphorylation by the Hippo pathway and subsequent cytoplasmic retention. Conversely, YAP can also augment Wnt signaling. Conditional knockout studies of the Hippo component Sav in mouse hearts led to increased Wnt signaling through direct interaction between unphosphorylated YAP and β-catenin on Wnt target genes (Heallen et al., 2011). Similarly, conditional intestinal knockouts of Mst1/2 also resulted in Wnt target gene expression (Zhou et al., 2009). Taken in aggregate, these studies suggest context dependent inhibition and upregulation of Wnt by YAP/TAZ. Further confounding the issue is the involvement of other pathways. Xin and colleagues showed that constitutively active YAP mutants upregulated insulin-like growth factor (IGF) signaling pathway, which in turn upregulated the Wnt pathway (Xin et al., 2011). In many of the above cited studies, YAP/TAZ localization was an important component of Wnt regulation, again emphasizing that any alteration in YAP/TAZ regulation will have far reaching consequences.
The Wnt pathway also regulates the expression and degradation of YAP/TAZ. In two independent studies YAP has also been identified as a target of TCF/β-catenin through the use of ChIP (Bottomly et al., 2010; Konsavage et al., 2012). Curiously, YAP expression is reported to be insensitive to lithium chloride, a potent activator of TCF/β-catenin, further emphasizing the context sensitive nature of YAP/TAZ (Bottomly et al., 2010). While TAZ has not been identified as a transcriptional target of Wnt, a recent report identifies the Axin/APC/GSK3β complex and phospo-β-catenin as potent inducers of TAZ degradation (Azzolin et al., 2012). As a result, Wnt activation inhibits TAZ degradation and thus allows increased TAZ activity in a mechanism very similar to β-catenin in canonical Wnt signaling.
3.6 YAP/TAZ and other pathways
In addition to the extensive crosstalk described above, YAP/TAZ is implicated in several other proliferation and differentiation control pathways. While a full listing is outside the scope of this review, PI(3)K-Akt and Retinoblastoma (Rb) deserve special mention. Multiple studies have revealed Akt to have direct kinase activity on YAP (Basu et al., 2003; Ehsanian et al., 2010; Strano et al., 2001; Zhang et al., 2012b). This is debated, further suggesting that confounding factors such as cell/tissue type play a role (Dong et al., 2007; Zhao et al., 2007). However, the action of Akt on YAP is supported by a recent report of Yki activation in response to IGF signaling, which in mammals involves Akt (Strassburger et al., 2012). This may be part of a feedback loop, as YAP/Yki have also been reported to initiate IGF signaling (Strassburger et al., 2012; Xin et al., 2011). Further confounding the crosstalk between YAP and Akt is the downregulation of PTEN, an antagonist of PI(3)K-Akt signaling, by YAP mediated expression of miR-29 (Tumaneng et al., 2012).
Of particular relevance to the eye, recent results have documented crosstalk between Hippo and Retinoblastoma (Rb) pathways. Through shRNA screening, LATS2 was identified as a key component of Rb induced senescence (Tschop et al., 2011). Importantly, these results do not implicate YAP/TAZ as effectors of this response, as a constitutively active YAP did not produce the same result as LATS2 depletion. With the double mutant of Rb and Hippo pathways in Drosophilarbf/wts, retinal cells initially differentiate normally but eventually dedifferentiate (Nicolay et al., 2010). While the mechanism behind this is not well understood, the behavior was not preserved in single mutants, identifying a requirement for both pathways in maintaining terminal differentiation in the Drosophila retina. The importance of Hippo signaling in retinal differentiation is conserved in mammals, with overexpression or depletion of YAP in developing mouse retina shifting retinal cells towards overproliferation or premature differentiation, respectively (Zhang et al., 2012a). In keeping with the trend of Hippo being both a regulator and a target of other pathways, several Hippo family members (including Wts and Hpo, but not Yki) are transcriptional targets of the Rb pathway (Acharya et al., 2012). The transcription of Wts and Hpo is consistent with Rb acting as a tumor suppressor.
4. Conclusions
In this review we have attempted to highlight the important role of mechanotransduction in ocular biology and provide evidence for YAP/TAZ acting as signaling mediators. A key conclusion is that YAP/TAZ interaction with other signaling pathways depending on YAP/TAZ expression and localization, making YAP/TAZ orchestrating molecules in the coordination of numerous proliferation and differentiation pathways, in addition to the direct action on their transcriptional targets. Additionally, we have sought to emphasize potential intersections of YAP/TAZ with vision science and where we see promising areas of future research. We should note that even the extensive discussion above does not provide a full view of the complexity of YAP/TAZ and their numerous signaling partners. However, we hope this overview accentuates that the consequences of YAP/TAZ, likely due to their extensive crosstalk with multiple pathways, are extremely context dependent. For these reasons, we expect YAP/TAZ to have important and unexpected functionality in the specialized tissues of the eye, and are excited to see this new area of research explored.
Highlights.
We present examples of changing biophysical cues in the eye
We discuss the implications this may have on cellular function
We introduce the proteins YAP and TAZ as mediators of biophysical cueing
We provide a history of YAP, TAZ and their known signaling partners
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acharya P, Negre N, Johnston J, Wei Y, White KP, Henry RW, Arnosti DN. Evidence for autoregulation and cell signaling pathway regulation from genome-wide binding of the Drosophila retinoblastoma protein. G3 (Bethesda) 2012;2:1459–1472. doi: 10.1534/g3.112.004424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acott TS, Kelley MJ. Extracellular matrix in the trabecular meshwork. Exp Eye Res. 2008;86:543–561. doi: 10.1016/j.exer.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcon C, Zaromytidou AI, Xi Q, Gao S, Yu J, Fujisawa S, Barlas A, Miller AN, Manova-Todorova K, Macias MJ, Sapkota G, Pan D, Massague J. Nuclear CDKs drive Smad transcriptional activation and turnover in BMP and TGF-beta pathways. Cell. 2009;139:757–769. doi: 10.1016/j.cell.2009.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albon J, Karwatowski WS, Avery N, Easty DL, Duance VC. Changes in the collagenous matrix of the aging human lamina cribrosa. Br J Ophthalmol. 1995;79:368–375. doi: 10.1136/bjo.79.4.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albon J, Karwatowski WS, Easty DL, Sims TJ, Duance VC. Age related changes in the non-collagenous components of the extracellular matrix of the human lamina cribrosa. Br J Ophthalmol. 2000;84:311–317. doi: 10.1136/bjo.84.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allingham RR, de Kater AW, Ethier CR, Anderson PJ, Hertzmark E, Epstein DL. The relationship between pore density and outflow facility in human eyes. Invest Ophthalmol Vis Sci. 1992;33:1661–1669. [PubMed] [Google Scholar]
- Angus L, Moleirinho S, Herron L, Sinha A, Zhang X, Niestrata M, Dholakia K, Prystowsky MB, Harvey KF, Reynolds PA, Gunn-Moore FJ. Willin/FRMD6 expression activates the Hippo signaling pathway kinases in mammals and antagonizes oncogenic YAP. Oncogene. 2012;31:238–250. doi: 10.1038/onc.2011.224. [DOI] [PubMed] [Google Scholar]
- Aomatsu K, Arao T, Abe K, Kodama A, Sugioka K, Matsumoto K, Kudo K, Kimura H, Fujita Y, Hayashi H, Nagai T, Shimomura Y, Nishio K. Slug is upregulated during wound healing and regulates cellular phenotypes in corneal epithelial cells. Invest Ophthalmol Vis Sci. 2012;53:751–756. doi: 10.1167/iovs.11-8222. [DOI] [PubMed] [Google Scholar]
- Azzolin L, Zanconato F, Bresolin S, Forcato M, Basso G, Bicciato S, Cordenonsi M, Piccolo S. Role of TAZ as Mediator of Wnt Signaling. Cell. 2012 doi: 10.1016/j.cell.2012.11.027. [DOI] [PubMed] [Google Scholar]
- Barry ER, Camargo FD. The Hippo superhighway: signaling crossroads converging on the Hippo/Yap pathway in stem cells and development. Curr Opin Cell Biol. 2013;25:247–253. doi: 10.1016/j.ceb.2012.12.006. [DOI] [PubMed] [Google Scholar]
- Barry ER, Morikawa T, Butler BL, Shrestha K, de la Rosa R, Yan KS, Fuchs CS, Magness ST, Smits R, Ogino S, Kuo CJ, Camargo FD. Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature. 2012 doi: 10.1038/nature11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S, Totty NF, Irwin MS, Sudol M, Downward J. Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14-3-3 and attenuation of p73-mediated apoptosis. Mol Cell. 2003;11:11–23. doi: 10.1016/s1097-2765(02)00776-1. [DOI] [PubMed] [Google Scholar]
- Bhatt K, Gong H, Freddo TF. Freeze-fracture studies of interendothelial junctions in the angle of the human eye. Invest Ophthalmol Vis Sci. 1995;36:1379–1389. [PubMed] [Google Scholar]
- Blanpain C, Horsley V, Fuchs E. Epithelial stem cells: turning over new leaves. Cell. 2007;128:445–458. doi: 10.1016/j.cell.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggiano JC, Fehon RG. Growth control by committee: intercellular junctions, cell polarity, and the cytoskeleton regulate Hippo signaling. Dev Cell. 2012;22:695–702. doi: 10.1016/j.devcel.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottomly D, Kyler SL, McWeeney SK, Yochum GS. Identification of {beta}-catenin binding regions in colon cancer cells using ChIP-Seq. Nucleic Acids Res. 2010;38:5735–5745. doi: 10.1093/nar/gkq363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussommier-Calleja A, Bertrand J, Woodward DF, Ethier CR, Stamer WD, Overby DR. Pharmacologic manipulation of conventional outflow facility in ex vivo mouse eyes. Invest Ophthalmol Vis Sci. 2012;53:5838–5845. doi: 10.1167/iovs.12-9923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunsmann C, Hammer CM, Rheinlaender J, Kruse FE, Schaffer TE, Schlotzer-Schrehardt U. Evaluation of lamina cribrosa and peripapillary sclera stiffness in pseudoexfoliation and normal eyes by atomic force microscopy. Invest Ophthalmol Vis Sci. 2012;53:2960–2967. doi: 10.1167/iovs.11-8409. [DOI] [PubMed] [Google Scholar]
- Callus BA, Verhagen AM, Vaux DL. Association of mammalian sterile twenty kinases, Mst1 and Mst2, with hSalvador via C-terminal coiled-coil domains, leads to its stabilization and phosphorylation. FEBS J. 2006;273:4264–4276. doi: 10.1111/j.1742-4658.2006.05427.x. [DOI] [PubMed] [Google Scholar]
- Camras LJ, Stamer WD, Epstein D, Gonzalez P, Yuan F. Differential effects of trabecular meshwork stiffness on outflow facility in normal human and porcine eyes. Invest Ophthalmol Vis Sci. 2012;53:5242–5250. doi: 10.1167/iovs.12-9825. [DOI] [PubMed] [Google Scholar]
- Chan EH, Nousiainen M, Chalamalasetty RB, Schafer A, Nigg EA, Sillje HH. The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene. 2005;24:2076–2086. doi: 10.1038/sj.onc.1208445. [DOI] [PubMed] [Google Scholar]
- Chan SW, Lim CJ, Chong YF, Pobbati AV, Huang C, Hong W. Hippo pathway-independent restriction of TAZ and YAP by angiomotin. J Biol Chem. 2011;286:7018–7026. doi: 10.1074/jbc.C110.212621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow A, Hao Y, Yang X. Molecular characterization of human homologs of yeast MOB1. Int J Cancer. 2010;126:2079–2089. doi: 10.1002/ijc.24878. [DOI] [PubMed] [Google Scholar]
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Davis JT, Wen Q, Janmey PA, Otteson DC, Foster WJ. Muller cell expression of genes implicated in proliferative vitreoretinopathy is influenced by substrate elastic modulus. Invest Ophthalmol Vis Sci. 2012;53:3014–3019. doi: 10.1167/iovs.11-8450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daxer A, Fratzl P. Collagen fibril orientation in the human corneal stroma and its implication in keratoconus. Invest Ophthalmol Vis Sci. 1997;38:121–129. [PubMed] [Google Scholar]
- de Iongh RU, Abud HE, Hime GR. WNT/Frizzled signaling in eye development and disease. Front Biosci. 2006;11:2442–2464. doi: 10.2741/1982. [DOI] [PubMed] [Google Scholar]
- de Iongh RU, Wederell E, Lovicu FJ, McAvoy JW. Transforming growth factor-beta-induced epithelial-mesenchymal transition in the lens: a model for cataract formation. Cells Tissues Organs. 2005;179:43–55. doi: 10.1159/000084508. [DOI] [PubMed] [Google Scholar]
- Dielemans I, de Jong PT, Stolk R, Vingerling JR, Grobbee DE, Hofman A. Primary open-angle glaucoma, intraocular pressure, and diabetes mellitus in the general elderly population. The Rotterdam Study. Ophthalmology. 1996;103:1271–1275. doi: 10.1016/s0161-6420(96)30511-3. [DOI] [PubMed] [Google Scholar]
- Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, Gayyed MF, Anders RA, Maitra A, Pan D. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreier B, Raghunathan VK, Russell P, Murphy CJ. Focal adhesion kinase knockdown modulates the response of human corneal epithelial cells to topographic cues. Acta Biomater. 2012;8:4285–4294. doi: 10.1016/j.actbio.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn GA, Brown AF. Alignment of fibroblasts on grooved surfaces described by a simple geometric transformation. J Cell Sci. 1986;83:313–340. doi: 10.1242/jcs.83.1.313. [DOI] [PubMed] [Google Scholar]
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- Ehsanian R, Brown M, Lu H, Yang XP, Pattatheyil A, Yan B, Duggal P, Chuang R, Doondeea J, Feller S, Sudol M, Chen Z, Van Waes C. YAP dysregulation by phosphorylation or DeltaNp63-mediated gene repression promotes proliferation, survival and migration in head and neck cancer subsets. Oncogene. 2010;29:6160–6171. doi: 10.1038/onc.2010.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis JD, Evans JM, Ruta DA, Baines PS, Leese G, MacDonald TM, Morris AD. Glaucoma incidence in an unselected cohort of diabetic patients: is diabetes mellitus a risk factor for glaucoma? DARTS/MEMO collaboration. Diabetes Audit and Research in Tayside Study. Medicines Monitoring Unit. Br J Ophthalmol. 2000;84:1218–1224. doi: 10.1136/bjo.84.11.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrigno O, Lallemand F, Verrecchia F, L’Hoste S, Camonis J, Atfi A, Mauviel A. Yes-associated protein (YAP65) interacts with Smad7 and potentiates its inhibitory activity against TGF-beta/Smad signaling. Oncogene. 2002;21:4879–4884. doi: 10.1038/sj.onc.1205623. [DOI] [PubMed] [Google Scholar]
- Fisher RF. The elastic constants of the human lens. J Physiol. 1971;212:147–180. doi: 10.1113/jphysiol.1971.sp009315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RF. The influence of age on some ocular basement membranes. Eye (Lond) 1987;1 ( Pt 2):184–189. doi: 10.1038/eye.1987.35. [DOI] [PubMed] [Google Scholar]
- Fossdal R, Jonasson F, Kristjansdottir GT, Kong A, Stefansson H, Gosh S, Gulcher JR, Stefansson K. A novel TEAD1 mutation is the causative allele in Sveinsson’s chorioretinal atrophy (helicoid peripapillary chorioretinal degeneration) Hum Mol Genet. 2004;13:975–981. doi: 10.1093/hmg/ddh106. [DOI] [PubMed] [Google Scholar]
- Freese JL, Pino D, Pleasure SJ. Wnt signaling in development and disease. Neurobiol Dis. 2010;38:148–153. doi: 10.1016/j.nbd.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchshofer R. The pathogenic role of transforming growth factor-beta2 in glaucomatous damage to the optic nerve head. Exp Eye Res. 2011;93:165–169. doi: 10.1016/j.exer.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Fuchshofer R, Tamm ER. The role of TGF-beta in the pathogenesis of primary open-angle glaucoma. Cell Tissue Res. 2012;347:279–290. doi: 10.1007/s00441-011-1274-7. [DOI] [PubMed] [Google Scholar]
- Fuchshofer R, Welge-Lussen U, Lutjen-Drecoll E, Birke M. Biochemical and morphological analysis of basement membrane component expression in corneoscleral and cribriform human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2006;47:794–801. doi: 10.1167/iovs.05-0292. [DOI] [PubMed] [Google Scholar]
- Gan XQ, Wang JY, Xi Y, Wu ZL, Li YP, Li L. Nuclear Dvl, c-Jun, beta-catenin, and TCF form a complex leading to stabilization of beta-catenin-TCF interaction. J Cell Biol. 2008;180:1087–1100. doi: 10.1083/jcb.200710050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genevet A, Wehr MC, Brain R, Thompson BJ, Tapon N. Kibra is a regulator of the Salvador/Warts/Hippo signaling network. Dev Cell. 2010;18:300–308. doi: 10.1016/j.devcel.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldich Y, Marcovich AL, Barkana Y, Mandel Y, Hirsh A, Morad Y, Avni I, Zadok D. Clinical and corneal biomechanical changes after collagen cross-linking with riboflavin and UV irradiation in patients with progressive keratoconus: results after 2 years of follow-up. Cornea. 2012;31:609–614. doi: 10.1097/ICO.0b013e318226bf4a. [DOI] [PubMed] [Google Scholar]
- Gong H, Ruberti J, Overby D, Johnson M, Freddo TF. A new view of the human trabecular meshwork using quick-freeze, deep-etch electron microscopy. Exp Eye Res. 2002;75:347–358. [PubMed] [Google Scholar]
- Gottanka J, Johnson DH, Martus P, Lutjen-Drecoll E. Severity of optic nerve damage in eyes with POAG is correlated with changes in the trabecular meshwork. Journal of glaucoma. 1997;6:123–132. [PubMed] [Google Scholar]
- Goulev Y, Fauny JD, Gonzalez-Marti B, Flagiello D, Silber J, Zider A. SCALLOPED interacts with YORKIE, the nuclear effector of the hippo tumor-suppressor pathway in Drosophila. Curr Biol. 2008;18:435–441. doi: 10.1016/j.cub.2008.02.034. [DOI] [PubMed] [Google Scholar]
- Grierson I, Lee WR, Abraham S, Howes RC. Associations between the cells of the walls of Schlemm’s canal. Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. Albrecht von Graefe’s archive for clinical and experimental ophthalmology. 1978;208:33–47. doi: 10.1007/BF00406980. [DOI] [PubMed] [Google Scholar]
- Grusche FA, Richardson HE, Harvey KF. Upstream regulation of the hippo size control pathway. Curr Biol. 2010;20:R574–582. doi: 10.1016/j.cub.2010.05.023. [DOI] [PubMed] [Google Scholar]
- Guilak F, Cohen DM, Estes BT, Gimble JM, Liedtke W, Chen CS. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell. 2009;5:17–26. doi: 10.1016/j.stem.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaratoglu F, Willecke M, Kango-Singh M, Nolo R, Hyun E, Tao C, Jafar-Nejad H, Halder G. The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat Cell Biol. 2006;8:27–36. doi: 10.1038/ncb1339. [DOI] [PubMed] [Google Scholar]
- Han H, Wecker T, Grehn F, Schlunck G. Elasticity-dependent modulation of TGF-beta responses in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2011;52:2889–2896. doi: 10.1167/iovs.10-6640. [DOI] [PubMed] [Google Scholar]
- Hao Y, Chun A, Cheung K, Rashidi B, Yang X. Tumor suppressor LATS1 is a negative regulator of oncogene YAP. J Biol Chem. 2008;283:5496–5509. doi: 10.1074/jbc.M709037200. [DOI] [PubMed] [Google Scholar]
- Harvey KF, Pfleger CM, Hariharan IK. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell. 2003;114:457–467. doi: 10.1016/s0092-8674(03)00557-9. [DOI] [PubMed] [Google Scholar]
- Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nature reviews. Cancer. 2013;13:246–257. doi: 10.1038/nrc3458. [DOI] [PubMed] [Google Scholar]
- Heallen T, Zhang M, Wang J, Bonilla-Claudio M, Klysik E, Johnson RL, Martin JF. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011;332:458–461. doi: 10.1126/science.1199010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hergovich A, Bichsel SJ, Hemmings BA. Human NDR kinases are rapidly activated by MOB proteins through recruitment to the plasma membrane and phosphorylation. Mol Cell Biol. 2005;25:8259–8272. doi: 10.1128/MCB.25.18.8259-8272.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hergovich A, Schmitz D, Hemmings BA. The human tumour suppressor LATS1 is activated by human MOB1 at the membrane. Biochem Biophys Res Commun. 2006;345:50–58. doi: 10.1016/j.bbrc.2006.03.244. [DOI] [PubMed] [Google Scholar]
- Hollman KW, O’Donnell M, Erpelding TN. Mapping elasticity in human lenses using bubble-based acoustic radiation force. Exp Eye Res. 2007;85:890–893. doi: 10.1016/j.exer.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong JH, Hwang ES, McManus MT, Amsterdam A, Tian Y, Kalmukova R, Mueller E, Benjamin T, Spiegelman BM, Sharp PA, Hopkins N, Yaffe MB. TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science. 2005;309:1074–1078. doi: 10.1126/science.1110955. [DOI] [PubMed] [Google Scholar]
- Horbelt D, Denkis A, Knaus P. A portrait of Transforming Growth Factor beta superfamily signalling: Background matters. Int J Biochem Cell Biol. 2012;44:469–474. doi: 10.1016/j.biocel.2011.12.013. [DOI] [PubMed] [Google Scholar]
- Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122:421–434. doi: 10.1016/j.cell.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Huang W, Lv X, Liu C, Zha Z, Zhang H, Jiang Y, Xiong Y, Lei QY, Guan KL. The N-terminal phosphodegron targets TAZ/WWTR1 protein for SCFbeta-TrCP-dependent degradation in response to phosphatidylinositol 3-kinase inhibition. J Biol Chem. 2012;287:26245–26253. doi: 10.1074/jbc.M112.382036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imajo M, Miyatake K, Iimura A, Miyamoto A, Nishida E. A molecular mechanism that links Hippo signalling to the inhibition of Wnt/beta-catenin signalling. EMBO J. 2012;31:1109–1122. doi: 10.1038/emboj.2011.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inomata H, Bill A, Smelser GK. Aqueous humor pathways through the trabecular meshwork and into Schlemm’s canal in the cynomolgus monkey (Macaca irus). An electron microscopic study. Am J Ophthalmol. 1972;73:760–789. doi: 10.1016/0002-9394(72)90394-7. [DOI] [PubMed] [Google Scholar]
- Inoue-Mochita M, Inoue T, Epstein DL, Blumer KJ, Rao PV. RGS2-deficient mice exhibit decreased intraocular pressure and increased retinal ganglion cell survival. Mol Vis. 2009;15:495–504. [PMC free article] [PubMed] [Google Scholar]
- Ishida W, Hamamoto T, Kusanagi K, Yagi K, Kawabata M, Takehara K, Sampath TK, Kato M, Miyazono K. Smad6 is a Smad1/5-induced smad inhibitor. Characterization of bone morphogenetic protein-responsive element in the mouse Smad6 promoter. J Biol Chem. 2000;275:6075–6079. doi: 10.1074/jbc.275.9.6075. [DOI] [PubMed] [Google Scholar]
- Itoh K, Brott BK, Bae GU, Ratcliffe MJ, Sokol SY. Nuclear localization is required for Dishevelled function in Wnt/beta-catenin signaling. Journal of biology. 2005;4:3. doi: 10.1186/jbiol20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer P, Lalane R, 3rd, Morris C, Challa P, Vann R, Rao PV. Autotaxin-lysophosphatidic acid axis is a novel molecular target for lowering intraocular pressure. PLoS One. 2012;7:e42627. doi: 10.1371/journal.pone.0042627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jester JV, Petroll WM, Cavanagh HD. Corneal stromal wound healing in refractive surgery: the role of myofibroblasts. Prog Retin Eye Res. 1999;18:311–356. doi: 10.1016/s1350-9462(98)00021-4. [DOI] [PubMed] [Google Scholar]
- Jia J, Zhang W, Wang B, Trinko R, Jiang J. The Drosophila Ste20 family kinase dMST functions as a tumor suppressor by restricting cell proliferation and promoting apoptosis. Genes Dev. 2003;17:2514–2519. doi: 10.1101/gad.1134003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. What controls aqueous humour outflow resistance? Exp Eye Res. 2006;82:545–557. doi: 10.1016/j.exer.2005.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M, Chan D, Read AT, Christensen C, Sit A, Ethier CR. The pore density in the inner wall endothelium of Schlemm’s canal of glaucomatous eyes. Invest Ophthalmol Vis Sci. 2002;43:2950–2955. [PubMed] [Google Scholar]
- Junglas B, Kuespert S, Seleem AA, Struller T, Ullmann S, Bosl M, Bosserhoff A, Kostler J, Wagner R, Tamm ER, Fuchshofer R. Connective tissue growth factor causes glaucoma by modifying the actin cytoskeleton of the trabecular meshwork. Am J Pathol. 2012;180:2386–2403. doi: 10.1016/j.ajpath.2012.02.030. [DOI] [PubMed] [Google Scholar]
- Justice RW, Zilian O, Woods DF, Noll M, Bryant PJ. The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 1995;9:534–546. doi: 10.1101/gad.9.5.534. [DOI] [PubMed] [Google Scholar]
- Kanai F, Marignani PA, Sarbassova D, Yagi R, Hall RA, Donowitz M, Hisaminato A, Fujiwara T, Ito Y, Cantley LC, Yaffe MB. TAZ: a novel transcriptional co-activator regulated by interactions with 14-3-3 and PDZ domain proteins. EMBO Journal. 2000;19:6778–6791. doi: 10.1093/emboj/19.24.6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kango-Singh M, Nolo R, Tao C, Verstreken P, Hiesinger PR, Bellen HJ, Halder G. Shar-pei mediates cell proliferation arrest during imaginal disc growth in Drosophila. Development. 2002;129:5719–5730. doi: 10.1242/dev.00168. [DOI] [PubMed] [Google Scholar]
- Karuri NW, Porri TJ, Albrecht RM, Murphy CJ, Nealey PF. Nano- and microscale holes modulate cell-substrate adhesion, cytoskeletal organization, and -beta 1 integrin localization in SV40 human corneal epithelial cells. Ieee T Nanobiosci. 2006;5:273–280. doi: 10.1109/tnb.2006.886570. [DOI] [PubMed] [Google Scholar]
- Kawashima M, Kawakita T, Higa K, Satake Y, Omoto M, Tsubota K, Shimmura S, Shimazaki J. Subepithelial corneal fibrosis partially due to epithelial-mesenchymal transition of ocular surface epithelium. Mol Vis. 2010;16:2727–2732. [PMC free article] [PubMed] [Google Scholar]
- Kim NG, Koh E, Chen X, Gumbiner BM. E-cadherin mediates contact inhibition of proliferation through Hippo signaling-pathway components. Proc Natl Acad Sci U S A. 2011;108:11930–11935. doi: 10.1073/pnas.1103345108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa M. A Sveinsson’s chorioretinal atrophy-associated missense mutation in mouse Tead1 affects its interaction with the co-factors YAP and TAZ. Biochem Biophys Res Commun. 2007;361:1022–1026. doi: 10.1016/j.bbrc.2007.07.129. [DOI] [PubMed] [Google Scholar]
- Klein BE, Klein R, Jensen SC. Open-angle glaucoma and older-onset diabetes. The Beaver Dam Eye Study. Ophthalmology. 1994;101:1173–1177. doi: 10.1016/s0161-6420(94)31191-2. [DOI] [PubMed] [Google Scholar]
- Komuro A, Nagai M, Navin NE, Sudol M. WW domain-containing protein YAP associates with ErbB-4 and acts as a co-transcriptional activator for the carboxyl-terminal fragment of ErbB-4 that translocates to the nucleus. J Biol Chem. 2003;278:33334–33341. doi: 10.1074/jbc.M305597200. [DOI] [PubMed] [Google Scholar]
- Konsavage WM, Jr, Kyler SL, Rennoll SA, Jin G, Yochum GS. Wnt/beta-catenin signaling regulates Yes-associated protein (YAP) gene expression in colorectal carcinoma cells. J Biol Chem. 2012;287:11730–11739. doi: 10.1074/jbc.M111.327767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni BB, Tighe PJ, Mohammed I, Yeung AM, Powe DG, Hopkinson A, Shanmuganathan VA, Dua HS. Comparative transcriptional profiling of the limbal epithelial crypt demonstrates its putative stem cell niche characteristics. BMC Genomics. 2010;11:526. doi: 10.1186/1471-2164-11-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lad EM, Cheshier SH, Kalani MY. Wnt-signaling in retinal development and disease. Stem Cells Dev. 2009;18:7–16. doi: 10.1089/scd.2008.0169. [DOI] [PubMed] [Google Scholar]
- Lai ZC, Wei X, Shimizu T, Ramos E, Rohrbaugh M, Nikolaidis N, Ho LL, Li Y. Control of cell proliferation and apoptosis by mob as tumor suppressor, mats. Cell. 2005;120:675–685. doi: 10.1016/j.cell.2004.12.036. [DOI] [PubMed] [Google Scholar]
- Last JA, Liliensiek SJ, Nealey PF, Murphy CJ. Determining the mechanical properties of human corneal basement membranes with atomic force microscopy. J Struct Biol. 2009;167:19–24. doi: 10.1016/j.jsb.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Last JA, Pan T, Ding Y, Reilly CM, Keller K, Acott TS, Fautsch MP, Murphy CJ, Russell P. Elastic modulus determination of normal and glaucomatous human trabecular meshwork. Invest Ophthalmol Vis Sci. 2011;52:2147–2152. doi: 10.1167/iovs.10-6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Last JA, Thomasy SM, Croasdale CR, Russell P, Murphy CJ. Compliance profile of the human cornea as measured by atomic force microscopy. Micron. 2012;43:1293–1298. doi: 10.1016/j.micron.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei QY, Zhang H, Zhao B, Zha ZY, Bai F, Pei XH, Zhao S, Xiong Y, Guan KL. TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the hippo pathway. Mol Cell Biol. 2008;28:2426–2436. doi: 10.1128/MCB.01874-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Guan JL, Chien S. Biochemistry and biomechanics of cell motility. Annual review of biomedical engineering. 2005;7:105–150. doi: 10.1146/annurev.bioeng.7.060804.100340. [DOI] [PubMed] [Google Scholar]
- Liliensiek SJ, Wood JA, Yong J, Auerbach R, Nealey PF, Murphy CJ. Modulation of human vascular endothelial cell behaviors by nanotopographic cues. Biomaterials. 2010;31:5418–5426. doi: 10.1016/j.biomaterials.2010.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liliom K, Guan Z, Tseng JL, Desiderio DM, Tigyi G, Watsky MA. Growth factor-like phospholipids generated after corneal injury. Am J Physiol. 1998;274:C1065–1074. doi: 10.1152/ajpcell.1998.274.4.C1065. [DOI] [PubMed] [Google Scholar]
- Liu CY, Zha ZY, Zhou X, Zhang H, Huang W, Zhao D, Li T, Chan SW, Lim CJ, Hong W, Zhao S, Xiong Y, Lei QY, Guan KL. The hippo tumor pathway promotes TAZ degradation by phosphorylating a phosphodegron and recruiting the SCF{beta}-TrCP E3 ligase. J Biol Chem. 2010a;285:37159–37169. doi: 10.1074/jbc.M110.152942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Xin Y, Ye F, Wang W, Lu Q, Kaplan HJ, Dean DC. Taz-tead1 links cell-cell contact to zeb1 expression, proliferation, and dedifferentiation in retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2010b;51:3372–3378. doi: 10.1167/iovs.09-4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutjen-Drecoll E. Morphological changes in glaucomatous eyes and the role of TGFbeta2 for the pathogenesis of the disease. Exp Eye Res. 2005;81:1–4. doi: 10.1016/j.exer.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Macdonald BT, He X. Frizzled and LRP5/6 Receptors for Wnt/beta-Catenin Signaling. Cold Spring Harb Perspect Biol. 2012:4. doi: 10.1101/cshperspect.a007880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney WM, Jr, Hong JH, Yaffe MB, Farrance IK. The transcriptional co-activator TAZ interacts differentially with transcriptional enhancer factor-1 (TEF-1) family members. Biochem J. 2005;388:217–225. doi: 10.1042/BJ20041434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao W, Millar JC, Wang WH, Silverman SM, Liu Y, Wordinger RJ, Rubin JS, Pang IH, Clark AF. Existence of the canonical wnt signaling pathway in the human trabecular meshwork. Invest Ophthalmol Vis Sci. 2012;53:7043–7051. doi: 10.1167/iovs.12-9664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez G, de Iongh RU. The lens epithelium in ocular health and disease. Int J Biochem Cell Biol. 2010;42:1945–1963. doi: 10.1016/j.biocel.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Massague J. TGFbeta signalling in context. Nat Rev Mol Cell Biol. 2012;13:616–630. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauviel A, Nallet-Staub F, Varelas X. Integrating developmental signals: a Hippo in the (path)way. Oncogene. 2012;31:1743–1756. doi: 10.1038/onc.2011.363. [DOI] [PubMed] [Google Scholar]
- McGinty SJ, Truscott RJ. Presbyopia: the first stage of nuclear cataract? Ophthalmic research. 2006;38:137–148. doi: 10.1159/000090645. [DOI] [PubMed] [Google Scholar]
- McKee CT, Last JA, Russell P, Murphy CJ. Indentation versus tensile measurements of Young’s modulus for soft biological tissues. Tissue engineering. Part B, Reviews. 2011a;17:155–164. doi: 10.1089/ten.teb.2010.0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee CT, Raghunathan VK, Nealey PF, Russell P, Murphy CJ. Topographic modulation of the orientation and shape of cell nuclei and their influence on the measured elastic modulus of epithelial cells. Biophys J. 2011b;101:2139–2146. doi: 10.1016/j.bpj.2011.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee CT, Wood JA, Shah NM, Fischer ME, Reilly CM, Murphy CJ, Russell P. The effect of biophysical attributes of the ocular trabecular meshwork associated with glaucoma on the cell response to therapeutic agents. Biomaterials. 2011c;32:2417–2423. doi: 10.1016/j.biomaterials.2010.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek KM, Tuft SJ, Huang Y, Gill PS, Hayes S, Newton RH, Bron AJ. Changes in collagen orientation and distribution in keratoconus corneas. Invest Ophthalmol Vis Sci. 2005;46:1948–1956. doi: 10.1167/iovs.04-1253. [DOI] [PubMed] [Google Scholar]
- Miller E, Yang J, Deran M, Wu C, Su AI, Bonamy GM, Liu J, Peters EC, Wu X. Identification of Serum-Derived Sphingosine-1-Phosphate as a Small Molecule Regulator of YAP. Chem Biol. 2012 doi: 10.1016/j.chembiol.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Mo JS, Yu FX, Gong R, Brown JH, Guan KL. Regulation of the Hippo-YAP pathway by protease-activated receptors (PARs) Genes Dev. 2012;26:2138–2143. doi: 10.1101/gad.197582.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat BA, Landman KA, Truscott RJ, Sweeney MH, Pope JM. Age-related changes in the kinetics of water transport in normal human lenses. Exp Eye Res. 1999;69:663–669. doi: 10.1006/exer.1999.0747. [DOI] [PubMed] [Google Scholar]
- Moleirinho S, Chang N, Sims AH, Tilston-Lunel AM, Angus L, Steele A, Boswell V, Barnett SC, Ormandy C, Faratian D, Gunn-Moore FJ, Reynolds PA. KIBRA exhibits MST-independent functional regulation of the Hippo signaling pathway in mammals. Oncogene. 2012 doi: 10.1038/onc.2012.196. [DOI] [PubMed] [Google Scholar]
- Morgan JT, Wood JA, Shah NM, Hughbanks ML, Russell P, Barakat AI, Murphy CJ. Integration of basal topographic cues and apical shear stress in vascular endothelial cells. Biomaterials. 2012;33:4126–4135. doi: 10.1016/j.biomaterials.2012.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JC, Jerdan JA, Dorman ME, Quigley HA. Structural proteins of the neonatal and adult lamina cribrosa. Arch Ophthalmol. 1989;107:1220–1224. doi: 10.1001/archopht.1989.01070020286040. [DOI] [PubMed] [Google Scholar]
- Murray LB, Lau YK, Yu Q. Merlin is a negative regulator of human melanoma growth. PLoS One. 2012;7:e43295. doi: 10.1371/journal.pone.0043295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai N, Klimava A, Lee WH, Izumi-Nagai K, Handa JT. CTGF is increased in basal deposits and regulates matrix production through the ERK (p42/p44mapk) MAPK and the p38 MAPK signaling pathways. Invest Ophthalmol Vis Sci. 2009;50:1903–1910. doi: 10.1167/iovs.08-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarajan RP, Zhang J, Li W, Chen Y. Regulation of Smad7 promoter by direct association with Smad3 and Smad4. J Biol Chem. 1999;274:33412–33418. doi: 10.1074/jbc.274.47.33412. [DOI] [PubMed] [Google Scholar]
- Nakatsu MN, Ding Z, Ng MY, Truong TT, Yu F, Deng SX. Wnt/beta-catenin signaling regulates proliferation of human cornea epithelial stem/progenitor cells. Invest Ophthalmol Vis Sci. 2011;52:4734–4741. doi: 10.1167/iovs.10-6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen C, Cone FE, Nguyen TD, Coudrillier B, Pease ME, Steinhart MR, Oglesby EN, Jefferys JL, Quigley HA. Studies of scleral biomechanical behavior related to susceptibility for retinal ganglion cell loss in experimental mouse glaucoma. Invest Ophthalmol Vis Sci. 2013;54:1767–1780. doi: 10.1167/iovs.12-10952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolay BN, Bayarmagnai B, Moon NS, Benevolenskaya EV, Frolov MV. Combined inactivation of pRB and hippo pathways induces dedifferentiation in the Drosophila retina. PLoS Genet. 2010;6:e1000918. doi: 10.1371/journal.pgen.1000918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka N, Inoue K, Adachi K, Kiyonari H, Ota M, Ralston A, Yabuta N, Hirahara S, Stephenson RO, Ogonuki N, Makita R, Kurihara H, Morin-Kensicki EM, Nojima H, Rossant J, Nakao K, Niwa H, Sasaki H. The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev Cell. 2009;16:398–410. doi: 10.1016/j.devcel.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Oakley C, Brunette DM. The sequence of alignment of microtubules, focal contacts and actin filaments in fibroblasts spreading on smooth and grooved titanium substrata. J Cell Sci. 1993;106 ( Pt 1):343–354. doi: 10.1242/jcs.106.1.343. [DOI] [PubMed] [Google Scholar]
- Oka T, Schmitt AP, Sudol M. Opposing roles of angiomotin-like-1 and zona occludens-2 on pro-apoptotic function of YAP. Oncogene. 2012;31:128–134. doi: 10.1038/onc.2011.216. [DOI] [PubMed] [Google Scholar]
- Omerovic J, Puggioni EM, Napoletano S, Visco V, Fraioli R, Frati L, Gulino A, Alimandi M. Ligand-regulated association of ErbB-4 to the transcriptional co-activator YAP65 controls transcription at the nuclear level. Exp Cell Res. 2004;294:469–479. doi: 10.1016/j.yexcr.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Ortiz D, Pinero D, Shabayek MH, Arnalich-Montiel F, Alio JL. Corneal biomechanical properties in normal, post-laser in situ keratomileusis, and keratoconic eyes. J Cataract Refract Surg. 2007;33:1371–1375. doi: 10.1016/j.jcrs.2007.04.021. [DOI] [PubMed] [Google Scholar]
- Osakada F, Ooto S, Akagi T, Mandai M, Akaike A, Takahashi M. Wnt signaling promotes regeneration in the retina of adult mammals. J Neurosci. 2007;27:4210–4219. doi: 10.1523/JNEUROSCI.4193-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota M, Sasaki H. Mammalian Tead proteins regulate cell proliferation and contact inhibition as transcriptional mediators of Hippo signaling. Development. 2008;135:4059–4069. doi: 10.1242/dev.027151. [DOI] [PubMed] [Google Scholar]
- Pantalacci S, Tapon N, Leopold P. The Salvador partner Hippo promotes apoptosis and cell-cycle exit in Drosophila. Nat Cell Biol. 2003;5:921–927. doi: 10.1038/ncb1051. [DOI] [PubMed] [Google Scholar]
- Park KS, Whitsett JA, Di Palma T, Hong JH, Yaffe MB, Zannini M. TAZ interacts with TTF-1 and regulates expression of surfactant protein-C. J Biol Chem. 2004;279:17384–17390. doi: 10.1074/jbc.M312569200. [DOI] [PubMed] [Google Scholar]
- Patey A, Savoldelli M, Pouliquen Y. Keratoconus and normal cornea: a comparative study of the collagenous fibers of the corneal stroma by image analysis. Cornea. 1984;3:119–124. [PubMed] [Google Scholar]
- Quigley HA. Open-angle glaucoma. N Engl J Med. 1993;328:1097–1106. doi: 10.1056/NEJM199304153281507. [DOI] [PubMed] [Google Scholar]
- Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42:297–319. doi: 10.1016/s0039-6257(97)00119-7. [DOI] [PubMed] [Google Scholar]
- Raghunathan VK, McKee CT, Tocce EJ, Nealey PF, Russell P, Murphy CJ. Nuclear and cellular alignment of primary corneal epithelial cells on topography. J Biomed Mater Res A. 2013a;101:1069–1079. doi: 10.1002/jbm.a.34417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghunathan VK, Morgan JT, Dreier B, Reilly CM, Thomasy SM, Wood JA, Ly I, Tuyen BC, Hughbanks M, Murphy CJ, Russell P. Role of substratum stiffness in modulating genes associated with extracellular matrix and mechanotransducers YAP and TAZ. Invest Ophthalmol Vis Sci. 2013b;54:378–386. doi: 10.1167/iovs.12-11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramrattan RS, van der Schaft TL, Mooy CM, de Bruijn WC, Mulder PG, de Jong PT. Morphometric analysis of Bruch’s membrane, the choriocapillaris, and the choroid in aging. Invest Ophthalmol Vis Sci. 1994;35:2857–2864. [PubMed] [Google Scholar]
- Rauskolb C, Pan G, Reddy BV, Oh H, Irvine KD. Zyxin links fat signaling to the hippo pathway. PLoS Biol. 2011;9:e1000624. doi: 10.1371/journal.pbio.1000624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohen JW, Lutjen-Drecoll E, Flugel C, Meyer M, Grierson I. Ultrastructure of the trabecular meshwork in untreated cases of primary open-angle glaucoma (POAG) Exp Eye Res. 1993;56:683–692. doi: 10.1006/exer.1993.1085. [DOI] [PubMed] [Google Scholar]
- Saika S. TGFbeta pathobiology in the eye. Lab Invest. 2006;86:106–115. doi: 10.1038/labinvest.3700375. [DOI] [PubMed] [Google Scholar]
- Saika S, Yamanaka O, Okada Y, Tanaka S, Miyamoto T, Sumioka T, Kitano A, Shirai K, Ikeda K. TGF beta in fibroproliferative diseases in the eye. Front Biosci (Schol Ed) 2009;1:376–390. doi: 10.2741/S32. [DOI] [PubMed] [Google Scholar]
- Sansores-Garcia L, Atkins M, Moya IM, Shahmoradgoli M, Tao C, Mills GB, Halder G. Mask is required for the activity of the Hippo pathway effector Yki/YAP. Curr Biol. 2013;23:229–235. doi: 10.1016/j.cub.2012.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansores-Garcia L, Bossuyt W, Wada K, Yonemura S, Tao C, Sasaki H, Halder G. Modulating F-actin organization induces organ growth by affecting the Hippo pathway. EMBO J. 2011;30:2325–2335. doi: 10.1038/emboj.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaguchi S, Twining SS, Yue BY, Chang SH, Zhou X, Loushin G, Sugar J, Feder RS. Alpha 2-macroglobulin levels in normal human and keratoconus corneas. Invest Ophthalmol Vis Sci. 1994;35:4008–4014. [PubMed] [Google Scholar]
- Sawaguchi S, Yue BY, Sugar J, Gilboy JE. Lysosomal enzyme abnormalities in keratoconus. Arch Ophthalmol. 1989;107:1507–1510. doi: 10.1001/archopht.1989.01070020581044. [DOI] [PubMed] [Google Scholar]
- Schlegelmilch K, Mohseni M, Kirak O, Pruszak J, Rodriguez JR, Zhou D, Kreger BT, Vasioukhin V, Avruch J, Brummelkamp TR, Camargo FD. Yap1 acts downstream of alpha-catenin to control epidermal proliferation. Cell. 2011;144:782–795. doi: 10.1016/j.cell.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlunck G, Han H, Wecker T, Kampik D, Meyer-ter-Vehn T, Grehn F. Substrate rigidity modulates cell matrix interactions and protein expression in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2008;49:262–269. doi: 10.1167/iovs.07-0956. [DOI] [PubMed] [Google Scholar]
- Shah S, Laiquzzaman M, Bhojwani R, Mantry S, Cunliffe I. Assessment of the biomechanical properties of the cornea with the ocular response analyzer in normal and keratoconic eyes. Invest Ophthalmol Vis Sci. 2007;48:3026–3031. doi: 10.1167/iovs.04-0694. [DOI] [PubMed] [Google Scholar]
- Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- Sidor CM, Brain R, Thompson BJ. Mask proteins are cofactors of Yorkie/YAP in the Hippo pathway. Curr Biol. 2013;23:223–228. doi: 10.1016/j.cub.2012.11.061. [DOI] [PubMed] [Google Scholar]
- Sigal IA, Ethier CR. Biomechanics of the optic nerve head. Exp Eye Res. 2009;88:799–807. doi: 10.1016/j.exer.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Silvis MR, Kreger BT, Lien WH, Klezovitch O, Rudakova GM, Camargo FD, Lantz DM, Seykora JT, Vasioukhin V. alpha-catenin is a tumor suppressor that controls cell accumulation by regulating the localization and activity of the transcriptional coactivator Yap1. Sci Signal. 2011;4:ra33. doi: 10.1126/scisignal.2001823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Mak KK, Topol L, Yun K, Hu J, Garrett L, Chen Y, Park O, Chang J, Simpson RM, Wang CY, Gao B, Jiang J, Yang Y. Mammalian Mst1 and Mst2 kinases play essential roles in organ size control and tumor suppression. Proc Natl Acad Sci U S A. 2010;107:1431–1436. doi: 10.1073/pnas.0911409107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spraul CW, Lang GE, Grossniklaus HE, Lang GK. Histologic and morphometric analysis of the choroid, Bruch’s membrane, and retinal pigment epithelium in postmortem eyes with age-related macular degeneration and histologic examination of surgically excised choroidal neovascular membranes. Surv Ophthalmol. 1999;44(Suppl 1):S10–32. doi: 10.1016/s0039-6257(99)00086-7. [DOI] [PubMed] [Google Scholar]
- Stamer WD, Read AT, Sumida GM, Ethier CR. Sphingosine-1-phosphate effects on the inner wall of Schlemm’s canal and outflow facility in perfused human eyes. Exp Eye Res. 2009;89:980–988. doi: 10.1016/j.exer.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strano S, Munarriz E, Rossi M, Castagnoli L, Shaul Y, Sacchi A, Oren M, Sudol M, Cesareni G, Blandino G. Physical interaction with Yes-associated protein enhances p73 transcriptional activity. J Biol Chem. 2001;276:15164–15173. doi: 10.1074/jbc.M010484200. [DOI] [PubMed] [Google Scholar]
- Strassburger K, Tiebe M, Pinna F, Breuhahn K, Teleman AA. Insulin/IGF signaling drives cell proliferation in part via Yorkie/YAP. Dev Biol. 2012;367:187–196. doi: 10.1016/j.ydbio.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Sumida GM, Stamer WD. S1P(2) receptor regulation of sphingosine-1-phosphate effects on conventional outflow physiology. Am J Physiol Cell Physiol. 2011;300:C1164–1171. doi: 10.1152/ajpcell.00437.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney MH, Truscott RJ. An impediment to glutathione diffusion in older normal human lenses: a possible precondition for nuclear cataract. Exp Eye Res. 1998;67:587–595. doi: 10.1006/exer.1998.0549. [DOI] [PubMed] [Google Scholar]
- Tamm ER, Fuchshofer R. What increases outflow resistance in primary open-angle glaucoma? Surv Ophthalmol. 2007;52(Suppl 2):S101–104. doi: 10.1016/j.survophthal.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Tapon N, Harvey KF, Bell DW, Wahrer DC, Schiripo TA, Haber DA, Hariharan IK. salvador Promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell. 2002;110:467–478. doi: 10.1016/s0092-8674(02)00824-3. [DOI] [PubMed] [Google Scholar]
- Terai N, Spoerl E, Haustein M, Hornykewycz K, Haentzschel J, Pillunat LE. Diabetes mellitus affects biomechanical properties of the optic nerve head in the rat. Ophthalmic research. 2012;47:189–194. doi: 10.1159/000331990. [DOI] [PubMed] [Google Scholar]
- Thomasy SM, Wood JA, Kass PH, Murphy CJ, Russell P. Substratum stiffness and latrunculin B regulate matrix gene and protein expression in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2012;53:952–958. doi: 10.1167/iovs.11-8526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton IL, Dupps WJ, Roy AS, Krueger RR. Biomechanical effects of intraocular pressure elevation on optic nerve/lamina cribrosa before and after peripapillary scleral collagen cross-linking. Invest Ophthalmol Vis Sci. 2009;50:1227–1233. doi: 10.1167/iovs.08-1960. [DOI] [PubMed] [Google Scholar]
- Tokumura A, Taira S, Kikuchi M, Tsutsumi T, Shimizu Y, Watsky MA. Lysophospholipids and lysophospholipase D in rabbit aqueous humor following corneal injury. Prostaglandins Other Lipid Mediat. 2012;97:83–89. doi: 10.1016/j.prostaglandins.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Tschop K, Conery AR, Litovchick L, Decaprio JA, Settleman J, Harlow E, Dyson N. A kinase shRNA screen links LATS2 and the pRB tumor suppressor. Genes Dev. 2011;25:814–830. doi: 10.1101/gad.2000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumaneng K, Schlegelmilch K, Russell RC, Yimlamai D, Basnet H, Mahadevan N, Fitamant J, Bardeesy N, Camargo FD, Guan KL. YAP mediates crosstalk between the Hippo and PI(3)K-TOR pathways by suppressing PTEN via miR-29. Nat Cell Biol. 2012;14:1322–1329. doi: 10.1038/ncb2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udan RS, Kango-Singh M, Nolo R, Tao C, Halder G. Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nat Cell Biol. 2003;5:914–920. doi: 10.1038/ncb1050. [DOI] [PubMed] [Google Scholar]
- Uttayarat P, Chen M, Li M, Allen FD, Composto RJ, Lelkes PI. Microtopography and flow modulate the direction of endothelial cell migration. Am J Physiol Heart Circ Physiol. 2008;294:H1027–1035. doi: 10.1152/ajpheart.00816.2007. [DOI] [PubMed] [Google Scholar]
- Varelas X, Miller BW, Sopko R, Song S, Gregorieff A, Fellouse FA, Sakuma R, Pawson T, Hunziker W, McNeill H, Wrana JL, Attisano L. The Hippo pathway regulates Wnt/beta-catenin signaling. Dev Cell. 2010a;18:579–591. doi: 10.1016/j.devcel.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Varelas X, Sakuma R, Samavarchi-Tehrani P, Peerani R, Rao BM, Dembowy J, Yaffe MB, Zandstra PW, Wrana JL. TAZ controls Smad nucleocytoplasmic shuttling and regulates human embryonic stem-cell self-renewal. Nat Cell Biol. 2008;10:837–848. doi: 10.1038/ncb1748. [DOI] [PubMed] [Google Scholar]
- Varelas X, Samavarchi-Tehrani P, Narimatsu M, Weiss A, Cockburn K, Larsen BG, Rossant J, Wrana JL. The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-beta-SMAD pathway. Dev Cell. 2010b;19:831–844. doi: 10.1016/j.devcel.2010.11.012. [DOI] [PubMed] [Google Scholar]
- von der Mark K, Park J, Bauer S, Schmuki P. Nanoscale engineering of biomimetic surfaces: cues from the extracellular matrix. Cell Tissue Res. 2010;339:131–153. doi: 10.1007/s00441-009-0896-5. [DOI] [PubMed] [Google Scholar]
- Wada K, Itoga K, Okano T, Yonemura S, Sasaki H. Hippo pathway regulation by cell morphology and stress fibers. Development. 2011;138:3907–3914. doi: 10.1242/dev.070987. [DOI] [PubMed] [Google Scholar]
- Wang W, Huang J, Chen J. Angiomotin-like proteins associate with and negatively regulate YAP1. J Biol Chem. 2011;286:4364–4370. doi: 10.1074/jbc.C110.205401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Huang J, Wang X, Yuan J, Li X, Feng L, Park JI, Chen J. PTPN14 is required for the density-dependent control of YAP1. Genes Dev. 2012;26:1959–1971. doi: 10.1101/gad.192955.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WH, McNatt LG, Pang IH, Millar JC, Hellberg PE, Hellberg MH, Steely HT, Rubin JS, Fingert JH, Sheffield VC, Stone EM, Clark AF. Increased expression of the WNT antagonist sFRP-1 in glaucoma elevates intraocular pressure. J Clin Invest. 2008;118:1056–1064. doi: 10.1172/JCI33871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watsky MA. Lysophosphatidic acid, serum, and hyposmolarity activate Cl- currents in corneal keratocytes. Am J Physiol. 1995;269:C1385–1393. doi: 10.1152/ajpcell.1995.269.6.C1385. [DOI] [PubMed] [Google Scholar]
- Weeber HA, Eckert G, Pechhold W, van der Heijde RG. Stiffness gradient in the crystalline lens. Graefes Arch Clin Exp Ophthalmol. 2007;245:1357–1366. doi: 10.1007/s00417-007-0537-1. [DOI] [PubMed] [Google Scholar]
- Wei X, Shimizu T, Lai ZC. Mob as tumor suppressor is activated by Hippo kinase for growth inhibition in Drosophila. EMBO J. 2007;26:1772–1781. doi: 10.1038/sj.emboj.7601630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde GS, Burd HJ, Judge SJ. Shear modulus data for the human lens determined from a spinning lens test. Exp Eye Res. 2012;97:36–48. doi: 10.1016/j.exer.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler M, Chai D, Kriling S, Nien CJ, Brown DJ, Jester B, Juhasz T, Jester JV. Nonlinear optical macroscopic assessment of 3-D corneal collagen organization and axial biomechanics. Invest Ophthalmol Vis Sci. 2011;52:8818–8827. doi: 10.1167/iovs.11-8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolffsohn JS, Safeen S, Shah S, Laiquzzaman M. Changes of corneal biomechanics with keratoconus. Cornea. 2012;31:849–854. doi: 10.1097/ICO.0b013e318243e42d. [DOI] [PubMed] [Google Scholar]
- Wood JA, McKee CT, Thomasy SM, Fischer ME, Shah NM, Murphy CJ, Russell P. Substratum compliance regulates human trabecular meshwork cell behaviors and response to latrunculin B. Invest Ophthalmol Vis Sci. 2011a;52:9298–9303. doi: 10.1167/iovs.11-7857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JA, Shah NM, McKee CT, Hughbanks ML, Liliensiek SJ, Russell P, Murphy CJ. The role of substratum compliance of hydrogels on vascular endothelial cell behavior. Biomaterials. 2011b;32:5056–5064. doi: 10.1016/j.biomaterials.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Huang J, Dong J, Pan D. hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell. 2003;114:445–456. doi: 10.1016/s0092-8674(03)00549-x. [DOI] [PubMed] [Google Scholar]
- Wu S, Liu Y, Zheng Y, Dong J, Pan D. The TEAD/TEF family protein Scalloped mediates transcriptional output of the Hippo growth-regulatory pathway. Dev Cell. 2008;14:388–398. doi: 10.1016/j.devcel.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Xiao L, Chen Y, Ji M, Dong J. KIBRA regulates Hippo signaling activity via interactions with large tumor suppressor kinases. J Biol Chem. 2011;286:7788–7796. doi: 10.1074/jbc.M110.173468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin M, Kim Y, Sutherland LB, Qi X, McAnally J, Schwartz RJ, Richardson JA, Bassel-Duby R, Olson EN. Regulation of insulin-like growth factor signaling by Yap governs cardiomyocyte proliferation and embryonic heart size. Sci Signal. 2011;4:ra70. doi: 10.1126/scisignal.2002278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu KP, Yin J, Yu FS. Lysophosphatidic acid promoting corneal epithelial wound healing by transactivation of epidermal growth factor receptor. Invest Ophthalmol Vis Sci. 2007;48:636–643. doi: 10.1167/iovs.06-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Wang W, Zhang S, Stewart RA, Yu W. Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development. 1995;121:1053–1063. doi: 10.1242/dev.121.4.1053. [DOI] [PubMed] [Google Scholar]
- Yagi R, Chen LF, Shigesada K, Murakami Y, Ito Y. A WW domain-containing yes-associated protein (YAP) is a novel transcriptional co-activator. EMBO J. 1999;18:2551–2562. doi: 10.1093/emboj/18.9.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z, Tong Y, Zhu H, Watsky MA. ClC-3 is required for LPA-activated Cl- current activity and fibroblast-to-myofibroblast differentiation. Am J Physiol Cell Physiol. 2008;294:C535–542. doi: 10.1152/ajpcell.00291.2007. [DOI] [PubMed] [Google Scholar]
- Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, Zhao J, Yuan H, Tumaneng K, Li H, Fu XD, Mills GB, Guan KL. Regulation of the Hippo-YAP Pathway by G-Protein-Coupled Receptor Signaling. Cell. 2012;150:780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Zheng Y, Dong J, Klusza S, Deng WM, Pan D. Kibra functions as a tumor suppressor protein that regulates Hippo signaling in conjunction with Merlin and Expanded. Dev Cell. 2010;18:288–299. doi: 10.1016/j.devcel.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi SK, Sullivan AJ, Medina R, Ito Y, van Wijnen AJ, Stein JL, Lian JB, Stein GS. Tyrosine phosphorylation controls Runx2-mediated subnuclear targeting of YAP to repress transcription. EMBO J. 2004;23:790–799. doi: 10.1038/sj.emboj.7600073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarbin MA. Current concepts in the pathogenesis of age-related macular degeneration. Arch Ophthalmol. 2004;122:598–614. doi: 10.1001/archopht.122.4.598. [DOI] [PubMed] [Google Scholar]
- Zhang H, Deo M, Thompson RC, Uhler MD, Turner DL. Negative regulation of Yap during neuronal differentiation. Dev Biol. 2012a;361:103–115. doi: 10.1016/j.ydbio.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Liu CY, Zha ZY, Zhao B, Yao J, Zhao S, Xiong Y, Lei QY, Guan KL. TEAD transcription factors mediate the function of TAZ in cell growth and epithelial-mesenchymal transition. J Biol Chem. 2009;284:13355–13362. doi: 10.1074/jbc.M900843200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Wu S, Xing D. Inhibition of Abeta(25–35)-induced cell apoptosis by low-power-laser-irradiation (LPLI) through promoting Akt-dependent YAP cytoplasmic translocation. Cell Signal. 2012b;24:224–232. doi: 10.1016/j.cellsig.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Zhang J, Smolen GA, Haber DA. Negative regulation of YAP by LATS1 underscores evolutionary conservation of the Drosophila Hippo pathway. Cancer Res. 2008a;68:2789–2794. doi: 10.1158/0008-5472.CAN-07-6205. [DOI] [PubMed] [Google Scholar]
- Zhang L, Ren F, Zhang Q, Chen Y, Wang B, Jiang J. The TEAD/TEF family of transcription factor Scalloped mediates Hippo signaling in organ size control. Dev Cell. 2008b;14:377–387. doi: 10.1016/j.devcel.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Bai H, David KK, Dong J, Zheng Y, Cai J, Giovannini M, Liu P, Anders RA, Pan D. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev Cell. 2010;19:27–38. doi: 10.1016/j.devcel.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Li L, Guan KL. Hippo signaling at a glance. J Cell Sci. 2010a;123:4001–4006. doi: 10.1242/jcs.069070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Li L, Lei Q, Guan KL. The Hippo-YAP pathway in organ size control and tumorigenesis: an updated version. Genes Dev. 2010b;24:862–874. doi: 10.1101/gad.1909210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Li L, Lu Q, Wang LH, Liu CY, Lei Q, Guan KL. Angiomotin is a novel Hippo pathway component that inhibits YAP oncoprotein. Genes Dev. 2011;25:51–63. doi: 10.1101/gad.2000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP) Genes Dev. 2010c;24:72–85. doi: 10.1101/gad.1843810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Li L, Wang L, Wang CY, Yu J, Guan KL. Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev. 2012;26:54–68. doi: 10.1101/gad.173435.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, Xie J, Ikenoue T, Yu J, Li L, Zheng P, Ye K, Chinnaiyan A, Halder G, Lai ZC, Guan KL. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Ye X, Yu J, Li L, Li W, Li S, Lin JD, Wang CY, Chinnaiyan AM, Lai ZC, Guan KL. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22:1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Conrad C, Xia F, Park JS, Payer B, Yin Y, Lauwers GY, Thasler W, Lee JT, Avruch J, Bardeesy N. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell. 2009;16:425–438. doi: 10.1016/j.ccr.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Medoff BD, Chen L, Li L, Zhang XF, Praskova M, Liu M, Landry A, Blumberg RS, Boussiotis VA, Xavier R, Avruch J. The Nore1B/Mst1 complex restrains antigen receptor-induced proliferation of naive T cells. Proc Natl Acad Sci U S A. 2008;105:20321–20326. doi: 10.1073/pnas.0810773105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Zhang Y, Wu H, Barry E, Yin Y, Lawrence E, Dawson D, Willis JE, Markowitz SD, Camargo FD, Avruch J. Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Yes-associated protein (Yap) overabundance. Proc Natl Acad Sci U S A. 2011;108:E1312–1320. doi: 10.1073/pnas.1110428108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou EH, Krishnan R, Stamer WD, Perkumas KM, Rajendran K, Nabhan JF, Lu Q, Fredberg JJ, Johnson M. Mechanical responsiveness of the endothelial cell of Schlemm’s canal: scope, variability and its potential role in controlling aqueous humour outflow. Journal of the Royal Society, Interface / the Royal Society. 2012;9:1144–1155. doi: 10.1098/rsif.2011.0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Sawaguchi S, Twining SS, Sugar J, Feder RS, Yue BY. Expression of degradative enzymes and protease inhibitors in corneas with keratoconus. Invest Ophthalmol Vis Sci. 1998;39:1117–1124. [PubMed] [Google Scholar]
- Zi Z, Chapnick DA, Liu X. Dynamics of TGF-beta/Smad signaling. FEBS Lett. 2012;586:1921–1928. doi: 10.1016/j.febslet.2012.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann DR, Fischer RW, Winterhalter KH, Witmer R, Vaughan L. Comparative studies of collagens in normal and keratoconus corneas. Exp Eye Res. 1988;46:431–442. doi: 10.1016/s0014-4835(88)80031-9. [DOI] [PubMed] [Google Scholar]