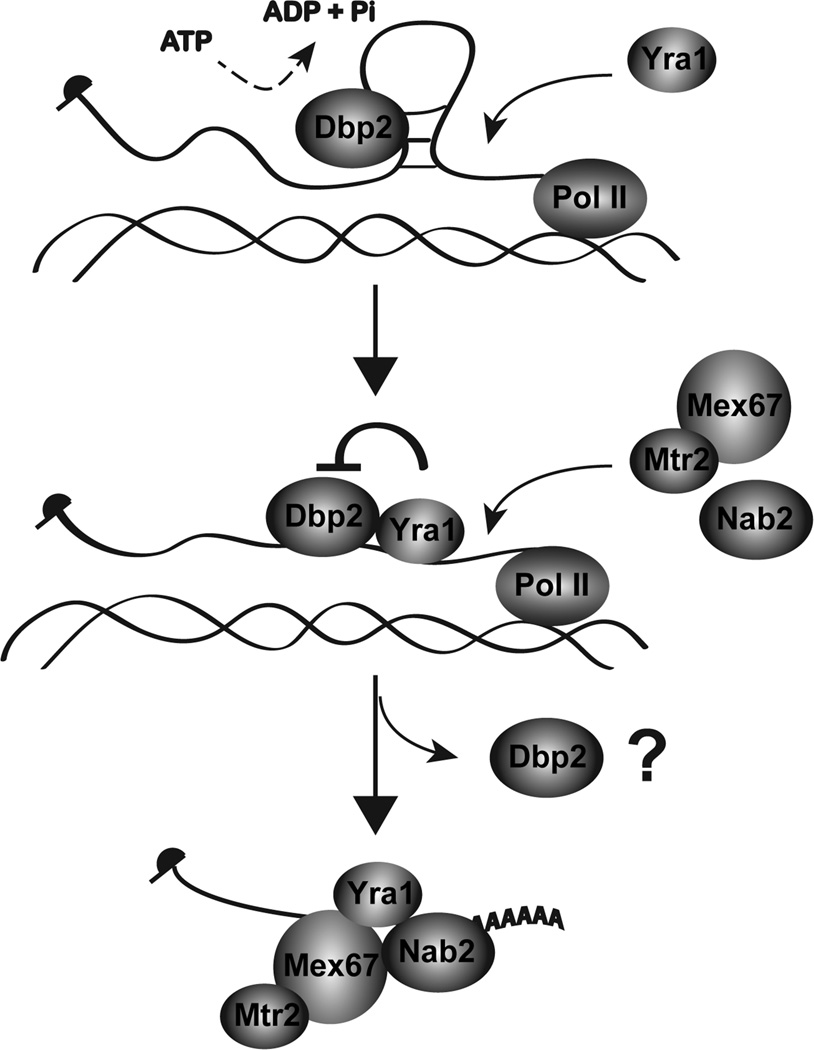
Figure 7. Model for Dbp2-dependent loading of RNA-binding proteins onto mRNA.
Yra1 is recruited to the actively transcribing loci through interacting with Sub2 or Pcf11 on the C-terminal domain of the RNA polymerase II 49; 81. However, structures of the nascent mRNA prevent association with Yra1. Dbp2 unwinds these structures co-transcriptionally in an ATP-dependent manner. This promotes mRNP assembly by facilitating loading of Yra1, Nab2, and Mex67 onto nascent mRNA. Mex67 is shown interacting with its heterodimerization partner, Mtr2 82. Yra1 then inhibits the helicase activity of Dbp2 to prevent further remodeling of the assembled mRNP and may also promote release of Dbp2 from the RNA. This constitutes a biochemical mechanism of RNA helicase unwinding and subsequent inhibition during co-transcriptional assembly of mRNAs in the nucleus.