Abstract
Background
This study compared the risk of fatal cerebrovascular accidents (CVA) in patients with early stage glottic larynx cancer receiving surgery or external beam radiation therapy (EBRT).
Methods and Materials
Using a competing risks survival analysis, we compared the risk of death due to CVA among patients with early stage glottic larynx cancer receiving surgery or EBRT in the SEER database.
Results
The cumulative incidence of fatal CVA at 15 years was higher in patients receiving EBRT (2.8 %; 95% CI 2.3%–3.4%) compared to surgery (1.5 %; 95% CI 0.8 %–2.3%, p= 0.024). In multivariable competing risks regression models, EBRT remained associated with an increased risk of fatal CVA compared to surgery (adjusted HR 1.75; 95% CI 1.04–2.96, p= 0.037).
Conclusion
Treatment of early stage glottic larynx cancer with EBRT was associated with a small increase in the risk of late fatal CVA events relative to surgery.
Keywords: Larynx Cancer, Radiation Therapy, Cerebrovascular Accidents
INTRODUCTION
Optimal treatment of early stage glottic larynx cancer provides high rates of tumor control, larynx preservation, and minimal morbidity. Standard treatment with radiotherapy, transoral laser microsurgery, or open partial laryngectomy is associated with excellent local control rates of 85–95%.1–3 External Beam Radiation Therapy (EBRT) is acutely well tolerated in most patients, and reports of severe treatment related complications are uncommon.1,4 However, the highly curable nature of this disease underscores the importance of studying long-term treatment related morbidity and mortality.
A growing body of evidence has suggested that receipt of head and neck EBRT may be associated with increased risk of vascular stenosis and subsequent CVA.5–7 However, previous studies have not explored long term cerebrovascular outcomes in the early stage patient population or have been limited by small sample size. Early stage glottic larynx cancer is unique among head and neck cancers in that definitive radiation therapy does not include elective treatment of the cervical lymph nodes, resulting in more limited radiation exposure to the carotid artery. Conventional EBRT consists of opposed lateral fields and does not typically consider or address radiation dose to the carotid arteries. While alternative treatment techniques such as the use of angled treatment fields or intensity modulated radiation therapy (IMRT) allow for reduction of carotid dose when compared to conventional treatment techniques, their role in routine clinical care remains controversial because of concerns that more conformal treatment may compromise excellent local control rates.8–10
CVA related outcomes in early stage glottic larynx cancer patients receiving EBRT are of particular interest due to excellent disease prognosis, and existing alternatives to conventional EBRT (i.e. surgery, or carotid sparing radiation techniques). Therefore we conducted a retrospective observational cohort study to compare the incidence of fatal CVA in patients with early stage glottic larynx cancer who received either surgery or EBRT.
METHODS AND MATERIALS
Data Source
This study used registry data from the Surveillance, Epidemiology, and End Results (SEER) database. The SEER program is a national cancer registry that collects information regarding incident cancer cases in registries which comprise approximately 26% of the US population.11 The SEER program includes data regarding cancer patients of all ages, in contrast to the linked SEER-Medicare database which is largely limited to older adult patients ≥ 65 years of age.12 This study was approved by the institutional review board of the University of Pennsylvania.
Study Cohort
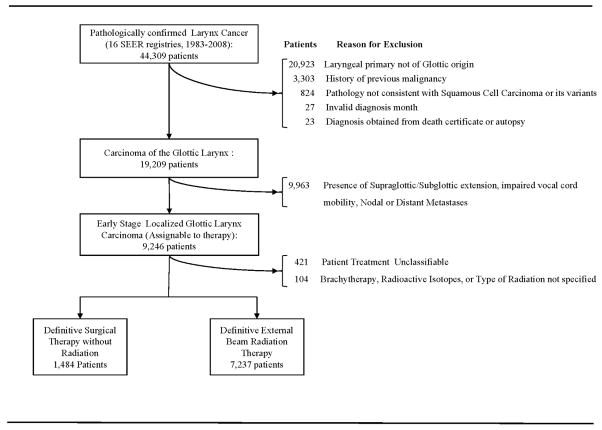
Eligible patients included individuals diagnosed with pathologically confirmed squamous cell carcinoma of the glottic larynx diagnosed between January 1, 1983 and December 31, 2008. Appendix Figure 1 illustrates definition of the study cohort and the reasons for exclusion. We limited our study population to patients with stage I disease. We identified 7,237 patients undergoing EBRT, and 1,484 patients undergoing surgery as initial management. Patients receiving both surgery and EBRT were excluded.
Appendix Figure 1.
Definition of Study Cohort (Online only)
Definition of Variables
The primary end point of this study was death from CVA. Cause of death is recorded for each individual followed longitudinally in the SEER database from death certificates, and is specified based upon International Classification of Diseases coding (ICD versions 8–10).
Receipt of surgical or radiation treatment was assigned based on treatment coding in SEER, which records the first course of cancer directed therapy initiated within one year of diagnosis. Surgical techniques are further specified in SEER and include local excision, laser surgery, and partial or total laryngectomy.
Patient characteristics included age at diagnosis, gender, race, ethnicity, and marital status. We staged patients according to the American Joint Commission on Cancer Staging Manual 7th edition from disease extent variables in SEER.13 Demographic characteristics included diagnosis year, SEER registry, and county or metropolitan area population. We used county level median household income as a proxy for individual socioeconomic status, because individual-level socioeconomic data are not available in SEER.
Statistical Analysis
We compared patient characteristics by treatment using chi-square statistics for categorical variables and t-tests for continuous variables. We analyzed survival and cumulative incidence of fatal CVA by treatment using a competing risks approach to account for the potential influence of death from other causes on the incidence of fatal CVA. Competing risks data analysis may be used to estimate the cumulative incidence of a specific event of interest when there are multiple potential dependent events of interest or competing outcomes. Under these conditions, a Kaplan-Meier approach may overestimate cumulative incidence because it censors competing risk events and assumes that all events are independent of one another. 14–16 Therefore we considered non-CVA related deaths to be competing risk events when estimating cumulative incidence functions of fatal CVA by treatment.
We compared cumulative incidence functions using k-sample test statistics, as described by Gray.17 In order to adjust for potential confounders, we used multivariable competing risks regression models to compare the risk of death due to CVA after treatment with surgery or EBRT.18 Covariates were selected based on clinical significance in prior studies or significance in univariate analysis. These included: age, gender, race, marital status, county or metropolitan area population, and median household income. We performed tests of interaction to examine potential effect modification of age, and year of diagnosis on the association of treatment with fatal CVA.
We determined number needed to harm from the inverse of attributable risk and report confidence intervals based on the Wilson score method.19 The number needed to harm may be interpreted as the average number of individuals receiving EBRT that will result in one excess fatal CVA compared to surgery at 15 years. As multivariable adjustment did not substantially attenuate the unadjusted estimate of the effect of radiotherapy on fatal CVA, we calculated the attributable risk of CVA associated with EBRT by subtracting the 15 year cumulative incidence of fatal CVA among patients receiving surgery from those receiving EBRT.20
We undertook a sensitivity analysis to further investigate patients’ baseline risk of vascular disease in which we compared the risk of death due to heart disease among the two treatment groups in a multivariable competing risks regression framework. Since EBRT in this setting does not result in radiation exposure to the heart, we expected no association between receipt of EBRT and death due to heart disease. Because baseline risk factors for heart disease and CVA are similar, this analysis served to further examine baseline risk of vascular disease in the two treatment groups.
Statistical analysis was performed using Stata version 12 (College Station, TX), and R version 2.7.2 (Vienna, Austria). Statistical significance was set at 0.05, and all tests were 2-tailed.
RESULTS
Patient Characteristics
The median follow-up time for the study population was 5.3 years (interquartile range 2.4–9.4 years). Characteristics of patients receiving surgery (n= 1,484) and EBRT (n= 7,237) are described in Table 1. The two treatment groups were similar with respect to patient and demographic characteristics. There was a statistically significant difference between the treatment groups in mean age (EBRT: 65.3 yrs, Surgery: 64.5 yrs; p= 0.01) and race.
Table 1.
Patient Characteristics by Treatment Group
| Characteristic | Surgery | EBRT | p-value |
|---|---|---|---|
| Treatment Received | 1,484 (17.0) | 7,237 (83.0) | |
| Patient Characteristics | |||
| Age at Diagnosis (years) | |||
| Mean (SD) | 64.5 (12.3) | 65.3 (11.3) | 0.01 |
| IQR | 16 (57–73) | 15 (58–73) | |
| Gender | |||
| Male | 1,285 (86.6) | 6,286 (86.9) | |
| Female | 199 (13.4) | 951 (13.1) | 0.78 |
| Race | |||
| White | 1,285 (86.6) | 6,171 (85.3) | |
| Black | 129 (8.7) | 723 (10.0) | |
| Other | 52 (3.5) | 318 (4.4) | |
| Unknown | 15 (1.0) | 20 (0.3) | < 0.001 |
| Hispanic Ethnicity | |||
| Hispanic | 104 (7.0) | 416 (5.8) | |
| Non-Hispanic | 1,380 (93.0) | 6,821 (94.2) | 0.06 |
| Marital Status | |||
| Married | 990 (66.7) | 4,841 (66.9) | |
| Not Married | 421 (28.4) | 2,126 (29.4) | |
| Unknown | 73 (4.9) | 270 (3.7) | 0.09 |
| Demographic Characteristics | |||
| Median Household Income ($, Thousands) | |||
| Mean (SD) | 46.8 (1.1) | 47.4 (1.1) | 0.03 |
| Population of county of residence | |||
| > 1,000,000 | 855 (57.6) | 4,457 (61.6) | |
| 250,000 to 1,000,000 | 299 (20.2) | 1,347 (18.6) | |
| 0 to 249,999 | 330 (22.2) | 1,429 (19.8) | |
| Unknown | * | * | 0.03 |
| Year of Diagnosis | |||
| 1983–1989 | 278 (18.7) | 1,010 (14.0) | |
| 1990–1999 | 443 (29.9) | 2,162 (29.9) | |
| 2000–2008 | 763 (51.4) | 4,065 (56.2) | < 0.001 |
Data are presented as number of patients(%).
Abbreviation: EBRT=External Beam Radiation Therapy, SD= Standard Deviation, IQR= Interquartile Range.
Number suppressed because of SEER confidentiality restrictions (cell size < 11).
Competing Risks Analysis of Death from CVA by Treatment
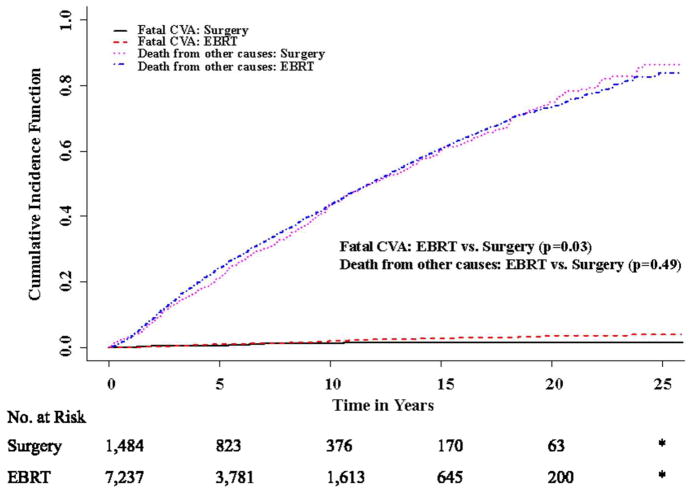
Accounting for competing risks, the unadjusted cumulative incidence of fatal CVA at 5, 10, and 15 years was 1.0 % (95% CI 0.8%–1.3%), 2.0 % (95% CI 1.6%–2.4%), and 2.8 % (95% CI 2.3%–3.4%) in the EBRT group and 0.6 % (95% CI 0.2%–1.0%), 1.4% (95% CI 0.7%–2.1%), and 1.5 % (95% CI 0.8%–2.3%) in the surgery group (Table 2, Fig 1; p= 0.02). The unadjusted 15 year cumulative incidence of death from non-CVA related causes was 60.7% (95% CI 58.9%– 62.5%) in patients receiving EBRT(p=0.49; Fig 1) and 60.4% (95% CI 56.7%– 64.2%) in patients receiving surgery. In unadjusted competing risks regression models, EBRT was associated with a significantly increased risk of fatal CVA compared to surgery [Hazard Ratio (HR) 1.72; 95% CI 1.02–2.89, p=0.04]. In multivariable competing risks regression models adjusted for patient and demographic characteristics, EBRT remained associated with an increased risk of fatal CVA compared to surgery (adjusted HR 1.75; 95% CI 1.04–2.96, p= 0.04; Table 3). Older age was also associated with an increased risk of CVA (year-on-year adjusted HR 1.07; 95% CI 1.06–1.09, p< 0.0001).
Table 2.
Cumulative Incidence of Fatal CVA and Heart Disease by Treatment
| Treatment | 5 year % (95% CI) | 10 year % (95% CI) | 15 year % (95% CI) | 20 year % (95% CI) | 25 year % (95% CI) |
|---|---|---|---|---|---|
| Death from CVA | |||||
| Surgery | 0.6 (0.2–1.0) | 1.4 (0.7–2.1) | 1.5 (0.8–2.3) | 1.5 (0.8–2.3) | 1.5 (0.8–2.3) |
| EBRT | 1.0 (0.8–1.3) | 2.0 (1.6–2.4) | 2.8 (2.3–3.4) | 3.7 (2.9–4.5) | 4.0 (3.0–4.9) |
| Death from Heart Disease | |||||
| Surgery | 5.7 (4.4–7.0) | 11.2 (9.2–13.2) | 14.8 (12.4–17.3) | 19.0 (15.8–22.1) | 21.6 (17.8–25.4) |
| EBRT | 5.2 (4.6–5.7) | 10.2 (9.3–11.0) | 14.3 (13.2–15.5) | 17.7 (16.2–19.2) | 20.2 (18.0–22.3) |
Note: All cumulative incidence estimates shown are accounting for competing risks.
Abbreviations: CI, Confidence Interval. EBRT, External beam radiation therapy. CVA, Cerebrovascular accidents.
Figure 1.
Cumulative Incidence of Fatal CVA and Death from Other Causes by Treatment
* Data suppressed because of SEER confidentiality restrictions (cell sizes < 11)
Table 3.
Adjusted Risk of Fatal CVA and Heart Disease by Treatment Group
| HR | 95% CI | P | |
|---|---|---|---|
| Risk of Fatal CVA (EBRT vs. Surgery) | |||
| Multivariable Competing Risks Regression Model | 1.75 | 1.04–2.96 | 0.0437 |
| Univariate Unadjusted Competing Risks Regression Model | 1.72 | 1.02–2.89 | 0.043 |
| Risk of Fatal Heart Disease (EBRT vs. Surgery) | |||
| Multivariable Competing Risks Regression Model | 0.912 | 0.77–1.09 | 0.30 |
| Overall Survival (EBRT vs. Surgery) | |||
| Cox Proportional Hazards Model | 1.03 | 0.905–1.13 | 0.48 |
Competing Risks Regression Model Adjusted for: Age at diagnosis, Gender, Median Houshold Income, Race, Ethnicity, Marital Status, Year of Diagnosis, and Population of County of Residence.
Abbreviations: EBRT= External Beam Radiation Therapy, CVA= Cerebrovascular accidents, HR= Hazard Ratio, CI= Confidence Interval
We also tested for interactions between age and treatment, as well as year of diagnosis and treatment on the occurrence of fatal CVA. Neither interaction term was statistically significant (p= 0.85 and p = 0.48, respectively).
Number Needed to Harm
The attributable risk of fatal CVA associated with exposure to EBRT was 1.3 % at 15 years, resulting in a number needed to harm of 77 (95% CI 50–100). In other words, on average the treatment of 77 early stage larynx cancer patients with EBRT would result in one excess fatal CVA compared to surgery at 15 years.
Competing Risks Analysis of Death from Heart Disease by Treatment
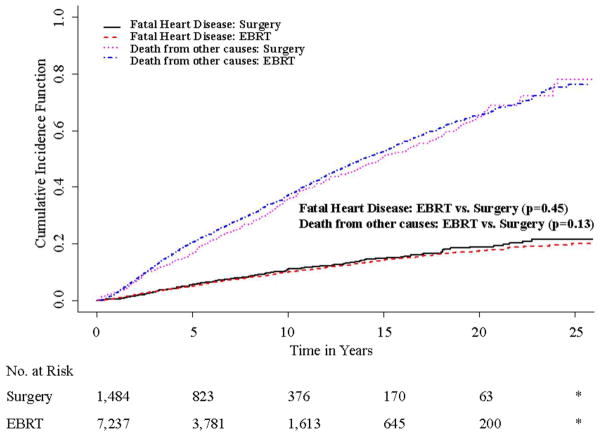
As a sensitivity analysis, we also compared the risk of death due to heart disease by treatment. Accounting for competing risks, the unadjusted 15 year cumulative incidence of death due to heart disease was 14.8 % (95% CI 12.4%–17.3%) in patients receiving surgery and 14.3 % (95% CI 13.2%–15.5%) in patients receiving EBRT (p=0.45, Fig 2). In multivariable competing risks regression models, EBRT was not associated with an increased risk of death due to heart disease (adjusted HR 0.912; 95% CI 0.77–1.09, p=0.3; Table 3).
Figure 2.
Cumulative Incidence of Fatal Heart Disease and Death from other Causes by Treatment
* Data suppressed because of SEER confidentiality restrictions (cell sizes < 11)
Overall Survival
There was no significant difference in overall survival between the treatment groups in either unadjusted analyses (data not shown) or in multivariable Cox proportional hazards models (Hazard Ratio (HR) 1.03; 95% CI 0.95–1.13; Table 3).
DISCUSSION
We undertook this study to compare the risk of fatal CVA in patients with early stage glottic larynx cancer who received either surgery or EBRT. Among patients in the SEER database, we found that treatment with EBRT was associated with a small increase in the risk of late fatal CVA events when compared to surgery.
Our results are consistent with and extend previously published studies reporting the association of head and neck EBRT with subsequent risk of CVA. A prior analysis of older head and neck cancer patients using SEER-Medicare data noted an increased risk of CVA events associated with receipt of EBRT compared to surgery (HR 1.50, 95% CI 1.18–1.90, p=0.0009). 5 However, the study did not report outcomes for patients diagnosed with primary laryngeal cancers and was limited to patients > 65 years of age. Another retrospective cohort study from the Netherlands estimated the risk of CVA from a sample of patients receiving EBRT alone for laryngeal and parotid malignancies.6 The authors compared the observed incidence of CVA in the study cohort with an expected CVA incidence rate from a geographically distant stroke registry, and reported an increased relative risk of CVA (RR 5.6, 95% CI 3.1–9.4) among patients receiving EBRT in the study. In contrast, our study made use of a large sample of patients treated for early stage glottic larynx cancer with and without exposure to EBRT, in order to examine the association of EBRT with the long term risk of subsequent fatal CVA.
Stroke related outcomes in patients with early stage glottic larynx cancer are of interest for several reasons. First, standard EBRT techniques for early stage glottic larynx cancer target the primary tumor and omit elective treatment of the neck because of the very low risk of lymph node involvement. Consequently a much shorter segment of the carotid artery receives radiation dose during EBRT when compared to other head and neck cancer patients requiring elective nodal treatment. In non-radiated individuals, vascular atherosclerosis most commonly occurs within 2 cm of the common carotid artery bifurcation which lies immediately adjacent to the glottic larynx.21 Our study findings suggest that even quite limited radiation exposure of the carotid arteries in this region significantly increases the subsequent risk of death due to CVA.
Second, several studies have investigated the use of advanced EBRT treatment techniques for patients with early stage glottic larynx cancer to allow for carotid artery dose reduction.8,9 Such techniques remain controversial because of concerns that more conformal EBRT may increase disease recurrence rates secondary to organ motion and contouring errors.9,10 Furthermore, there is no data currently available to inform estimates of dose response and a probable dose threshold for carotid injury. The results from our study underscore the potential importance of careful RT treatment planning and consideration of carotid dose reduction with the goal of reducing long term treatment related morbidity and mortality. Future studies should continue to investigate the use of conformal radiotherapy techniques in this setting to evaluate its safety and the potential reduction of treatment related toxicity such as CVA.
Third, advances in minimally invasive surgery such as the development of endoscopic laser microsurgery have allowed for high rates of local control as well as preservation of voice quality comparable to EBRT in patients with well-defined glottic carcinomas.2,22,23 Such techniques may allow for reduced treatment related morbidity in selected patients. Future evaluations of treatment related morbidity remain critical to answer questions of comparative effectiveness.
Finally, consensus guidelines recommend screening for asymptomatic carotid artery stenosis in patients with a carotid bruit, known atherosclerosis, or at least two of the following risk factors for CVA: hypertension, hyperlipidemia, tobacco use, and family history of CVA.24 EBRT is associated with a similar risk of CVA events compared to other established CVA risk factors (Relative Risk range 1.4–3.2); however, EBRT is not currently addressed in published screening recommendations.25 Furthermore, while there is growing evidence that head and neck EBRT is associated with an elevated risk of carotid stenosis, effective management strategies are not well established.26,27 Medical management of CVA risk factors may include the use of anti-platelet, anti-coagulation, anti-hypertensive, or lipid-lowering agents. However, additional studies are needed to evaluate the efficacy of such agents in preventing radiation-associated vascular disease. While small studies suggest that carotid endarterectomy (CEA) and carotid angioplasty with stenting (CAS) are feasible in previously radiated patients, further investigation is needed to evaluate the efficacy of these procedures in such patients compared to medical management alone.28–32
Our study has several limitations. First, as with any observational study, residual bias may remain even after adjusting for measured confounders.33 For example, our study lacks information regarding diabetes, tobacco use or other diagnosed comorbidities that are associated with the risk of CVA. To address this we conducted multivariable competing risks survival analyses, and also compared the risk of non-CVA related mortality by treatment. We found no significant differences observed between the treatment groups with respect to overall survival or death due to other causes. We also examined fatal heart disease because the underlying risk factors for CVA and heart disease are similar. We found no association between treatment and death due to heart disease, which suggests that unmeasured differences in baseline risk of vascular disease between the two patient groups would not explain our primary finding of an association of EBRT with CVA.
Second, prior studies have compared the reliability of death certificates relative to secondary expert review of medical records and report positive predictive values of 59–96% in suspected cases of CVA.34–36 While measurement error is a concern when using administrative data sets, these errors are likely to be non-differential between the treatment groups. Non-differential errors in sensitivity or specificity would bias observed study results toward the null hypothesis.35 Therefore, our estimates of fatal CVA in this study are conservative.
Third, SEER only records fatal CVA, rather than other non-fatal CVA events. Approximately 20% of all CVA events are acutely fatal; therefore, our study likely underestimates the total CVA-related disease burden in this study population.37 Fourth, our study cannot compare or adjust for local control rates between the treatment groups as this data is not available in SEER. However, expected local control rates would be high in both treatment groups. Finally, the data available in SEER do not specify radiation therapy details with respect to technique or dose delivered. However, radiation treatment techniques for early stage glottic larynx cancer have changed only minimally during the past few decades, with the exception of the very recent interest in the use of IMRT.
In conclusion, we found that treatment of early stage glottic larynx cancer with EBRT was associated with a small increase in the risk of late fatal CVA events relative to surgery. These results suggest the need for attention to carotid dose reduction through advanced conformal radiation treatment techniques, for further evaluation of modern surgical techniques, and for consideration of CVA prevention recommendations in patients receiving EBRT.
Acknowledgments
Funding: Dr. Bekelman is supported by an institutional Paul Celebresi National Cancer Institute Career Development Award (K12-CA076931).
This study used the linked Surveillance, Epidemiology, and End Results database. The authors acknowledge the efforts of the Applied Research Program, National Cancer Institute (NCI), the Office of Research, Development and Information, Information Management Services (IMS), Inc., and the SEER Program tumor registries in the creation of the SEER database.
Footnotes
Prior Presentation: This research was presented in part at the Multidisciplinary Head and Neck Cancer symposium January 26-28, 2012, Phoenix, Arizona and at the American Society for Radiation Oncology Annual Meeting October 28-31, 2012, Boston, MA.
Financial Disclosures: None
Disclaimer: The interpretation and reporting of these data are the sole responsibility of the authors.
Author Contributions: Dr. Swisher-McClure had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conception and design: Swisher-McClure, Bekelman
Collection and assembly of data: Swisher-McClure, Wan, and Bekelman
Manuscript Writing: All authors
Data analysis and interpretation: All authors
Financial Support: Bekelman
Provision of study materials or patients: Swisher-McClure
Final approval of manuscript: All authors
References
- 1.Mendenhall WM, Amdur RJ, Morris CG, et al. T1-T2N0 squamous cell carcinoma of the glottic larynx treated with radiation therapy. J Clin Oncol. 2001;19:4029–36. doi: 10.1200/JCO.2001.19.20.4029. [DOI] [PubMed] [Google Scholar]
- 2.Gallo A, de Vincentiis M, Manciocco V, et al. CO2 laser cordectomy for early-stage glottic carcinoma: a long-term follow-up of 156 cases. Laryngoscope. 2002;112:370–4. doi: 10.1097/00005537-200202000-00030. [DOI] [PubMed] [Google Scholar]
- 3.Giovanni A, Guelfucci B, Gras R, et al. Partial frontolateral laryngectomy with epiglottic reconstruction for management of early-stage glottic carcinoma. Laryngoscope. 2001;111:663–8. doi: 10.1097/00005537-200104000-00020. [DOI] [PubMed] [Google Scholar]
- 4.Garden AS, Forster K, Wong PF, et al. Results of radiotherapy for T2N0 glottic carcinoma: does the “2” stand for twice-daily treatment? Int J Radiat Oncol Biol Phys. 2003;55:322–8. doi: 10.1016/s0360-3016(02)03938-x. [DOI] [PubMed] [Google Scholar]
- 5.Smith GL, Smith BD, Buchholz TA, et al. Cerebrovascular disease risk in older head and neck cancer patients after radiotherapy. J Clin Oncol. 2008;26:5119–25. doi: 10.1200/JCO.2008.16.6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dorresteijn LD, Kappelle AC, Boogerd W, et al. Increased risk of ischemic stroke after radiotherapy on the neck in patients younger than 60 years. J Clin Oncol. 2002;20:282–8. doi: 10.1200/JCO.2002.20.1.282. [DOI] [PubMed] [Google Scholar]
- 7.Haynes JC, Machtay M, Weber RS, et al. Relative risk of stroke in head and neck carcinoma patients treated with external cervical irradiation. Laryngoscope. 2002;112:1883–7. doi: 10.1097/00005537-200210000-00034. [DOI] [PubMed] [Google Scholar]
- 8.Gomez D, Cahlon O, Mechalakos J, et al. An investigation of intensity-modulated radiation therapy versus conventional two-dimensional and 3D-conformal radiation therapy for early stage larynx cancer. Radiat Oncol. 2010;5:74. doi: 10.1186/1748-717X-5-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chera BS, Amdur RJ, Morris CG, et al. Carotid-sparing intensity-modulated radiotherapy for early-stage squamous cell carcinoma of the true vocal cord. Int J Radiat Oncol Biol Phys. 2010;77:1380–5. doi: 10.1016/j.ijrobp.2009.07.1687. [DOI] [PubMed] [Google Scholar]
- 10.Feigenberg SJ, Lango M, Nicolaou N, et al. Intensity-modulated radiotherapy for early larynx cancer: is there a role? Int J Radiat Oncol Biol Phys. 2007;68:2–3. doi: 10.1016/j.ijrobp.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Surveillance E, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database. Incidence - SEER 9 Regs Research Data, Nov 2010 Sub (1973–2008) National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2011, based on the November 2010 submission.
- 12.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40:IV-3-18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 13.American Joint Committee on Cancer. AJCC Cancer Staging Manual. 7. New York: Springer; 2010. [Google Scholar]
- 14.Satagopan JM, Ben-Porat L, Berwick M, et al. A note on competing risks in survival data analysis. Br J Cancer. 2004;91:1229–35. doi: 10.1038/sj.bjc.6602102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim HT. Cumulative incidence in competing risks data and competing risks regression analysis. Clin Cancer Res. 2007;13:559–65. doi: 10.1158/1078-0432.CCR-06-1210. [DOI] [PubMed] [Google Scholar]
- 16.Varadhan R, Weiss CO, Segal JB, et al. Evaluating health outcomes in the presence of competing risks: a review of statistical methods and clinical applications. Med Care. 2010;48:S96–105. doi: 10.1097/MLR.0b013e3181d99107. [DOI] [PubMed] [Google Scholar]
- 17.Gray RJ. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. The Annals of Statistics. 1988;16:1141–1154. [Google Scholar]
- 18.Fine JGR. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 19.Bender R. Calculating confidence intervals for the number needed to treat. Control Clin Trials. 2001;22:102–10. doi: 10.1016/s0197-2456(00)00134-3. [DOI] [PubMed] [Google Scholar]
- 20.Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. BMJ. 1995;310:452–4. doi: 10.1136/bmj.310.6977.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fisher M. Occlusion of the internal carotid artery. AMA Arch Neurol Psychiatry. 1951;65:346–77. doi: 10.1001/archneurpsyc.1951.02320030083009. [DOI] [PubMed] [Google Scholar]
- 22.Grant DG, Salassa JR, Hinni ML, et al. Transoral laser microsurgery for untreated glottic carcinoma. Otolaryngol Head Neck Surg. 2007;137:482–6. doi: 10.1016/j.otohns.2007.05.064. [DOI] [PubMed] [Google Scholar]
- 23.Tamura E, Kitahara S, Ogura M, et al. Voice quality after laser surgery or radiotherapy for T1a glottic carcinoma. Laryngoscope. 2003;113:910–4. doi: 10.1097/00005537-200305000-00025. [DOI] [PubMed] [Google Scholar]
- 24.Brott TG, Halperin JL, Abbara S, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery. Stroke. 2011;42:e420–63. [Google Scholar]
- 25.Wolf PA, D’Agostino RB, Belanger AJ, et al. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22:312–8. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- 26.Steele SR, Martin MJ, Mullenix PS, et al. Focused high-risk population screening for carotid arterial stenosis after radiation therapy for head and neck cancer. Am J Surg. 2004;187:594–8. doi: 10.1016/j.amjsurg.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 27.Brown PD, Foote RL, McLaughlin MP, et al. A historical prospective cohort study of carotid artery stenosis after radiotherapy for head and neck malignancies. Int J Radiat Oncol Biol Phys. 2005;63:1361–7. doi: 10.1016/j.ijrobp.2005.05.046. [DOI] [PubMed] [Google Scholar]
- 28.Kashyap VS, Moore WS, Quinones-Baldrich WJ. Carotid artery repair for radiation-associated atherosclerosis is a safe and durable procedure. J Vasc Surg. 1999;29:90–6. doi: 10.1016/s0741-5214(99)70351-4. discussion 97–9. [DOI] [PubMed] [Google Scholar]
- 29.Leseche G, Castier Y, Chataigner O, et al. Carotid artery revascularization through a radiated field. J Vasc Surg. 2003;38:244–50. doi: 10.1016/s0741-5214(03)00320-3. [DOI] [PubMed] [Google Scholar]
- 30.Rockman CB, Riles TS, Fisher FS, et al. The surgical management of carotid artery stenosis in patients with previous neck irradiation. Am J Surg. 1996;172:191–5. doi: 10.1016/S0002-9610(96)00150-X. [DOI] [PubMed] [Google Scholar]
- 31.Sadek M, Cayne NS, Shin HJ, et al. Safety and efficacy of carotid angioplasty and stenting for radiation-associated carotid artery stenosis. J Vasc Surg. 2009;50:1308–13. doi: 10.1016/j.jvs.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 32.Tallarita T, Oderich GS, Lanzino G, et al. Outcomes of carotid artery stenting versus historical surgical controls for radiation-induced carotid stenosis. J Vasc Surg. 2011;53:629-36 e1–5. doi: 10.1016/j.jvs.2010.09.056. [DOI] [PubMed] [Google Scholar]
- 33.Bosco JL, Silliman RA, Thwin SS, et al. A most stubborn bias: no adjustment method fully resolves confounding by indication in observational studies. J Clin Epidemiol. 2010;63:64–74. doi: 10.1016/j.jclinepi.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Halanych JH, Shuaib F, Parmar G, et al. Agreement on cause of death between proxies, death certificates, and clinician adjudicators in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. Am J Epidemiol. 2011;173:1319–26. doi: 10.1093/aje/kwr033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lloyd-Jones DM, Martin DO, Larson MG, et al. Accuracy of death certificates for coding coronary heart disease as the cause of death. Ann Intern Med. 1998;129:1020–6. doi: 10.7326/0003-4819-129-12-199812150-00005. [DOI] [PubMed] [Google Scholar]
- 36.Iso H, Jacobs DR, Jr, Goldman L. Accuracy of death certificate diagnosis of intracranial hemorrhage and nonhemorrhagic stroke. The Minnesota Heart Survey. Am J Epidemiol. 1990;132:993–8. doi: 10.1093/oxfordjournals.aje.a115742. [DOI] [PubMed] [Google Scholar]
- 37.Roger VL, Go AS, Lloyd-Jones DM, et al. Executive summary: heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:188–97. doi: 10.1161/CIR.0b013e3182456d46. [DOI] [PubMed] [Google Scholar]