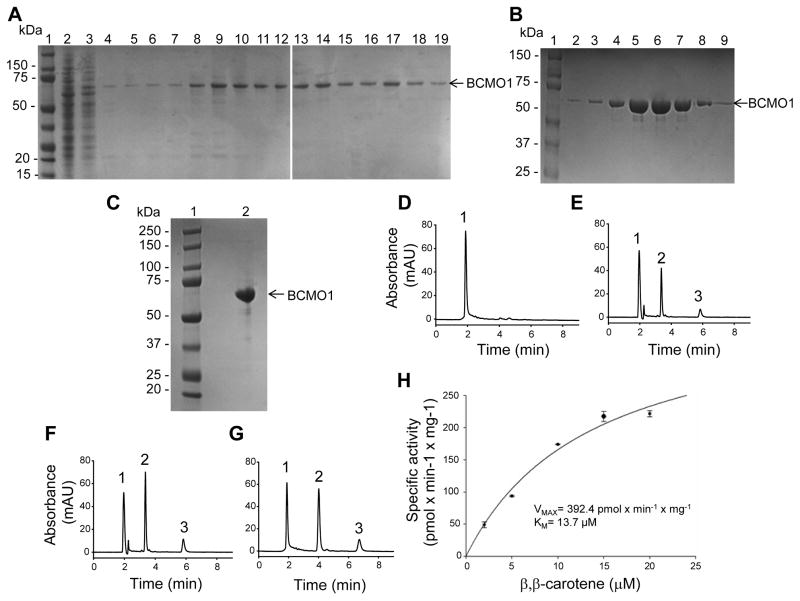
Figure 3. Purification of human BCMO1 and its enzymatic properties.
SDS-polyacrylamide gel electrophoresis of Co2+ metal affinity chromatography purified BCMO1 (A), further purification on size exclusion chromatography(B), and concentrated sample after size exclusion chromatography (C). Products formation from all-trans-β,β-carotene by BCMO1 after Co2+-column chromatography (E and F) compared with concentrated purified enzyme after size exclusion column chromatography (G). The enzyme was purified and assayed as described under “Materials and Methods”. 1, all-trans-β,β-carotene. 2, all-trans-retinal oxime (syn). 3, all-trans-retinal oxime (anti). (H) Talon purified recombinant human BCMO1 was incubated in the presence of increasing concentrations of β,β-carotene for 8 min at 28°C. Purified recombinant human BCMO1 displayed Michaelis-Menten kinetics.