Abstract
The effectiveness of highly active antiretroviral therapy (HAART) in preventing disease progression can be negatively influenced by the high prevalence of substance use among patients. Here, we quantify the effect of history of injection drug use and alcoholism on virologic and immunologic response to HAART. Clinical and survey data, collected at the start of HAART and at the interview date, were based on the study Longitudinal Investigations into Supportive and Ancillary Health Services (LISA) in British Columbia, Canada. Substance use was a three-level categorical variable, combining information on history of alcohol dependence and of injection drug use, defined as: no history of alcohol and injection drug use, history of alcohol or injection drug use and history of both alcohol and injection drug use. Virologic response (pVL) was defined by ≥2 log10 copy/mL drop in viral load. Immunologic response was defined as an increase in CD4 cell count percent of ≥100%. We used cumulative logit modeling for ordinal responses to address our objective. Of the 537 HIV-infected patients, 112 (21%) were characterized as having history of both alcohol and injection drug use, 173 (32%) were non adherent (<95%), 196 (36%) had CD4+/pVL+ (Best) response, 180 (34%) a CD4+/pVL− or a CD4−/pVL+ (Incomplete) response, and 161 (30%) a CD4−/pVL− (Worst) response. For individuals with history of both alcohol and injection drug use, the estimated probability of of Best, Incomplete and Worse responses, respectively. Screening and detection of substance dependence will identify individuals at high-risk for non-adherence and ideally prevent their HIV disease from progressing to advanced stages where HIV disease can become difficult to manage.
Keywords: Alcohol, Injection drug use, Adherence, HAART, HIV, Disease progression
INTRODUCTION
The primary goal of highly active antiretroviral therapy (HAART) is to achieve full and long-term suppression of viral replication, and thereby, to prevent the emergence of drug resistance and morbidity and mortality (Thompson et al., 2012). Unfortunately, the effectiveness of HAART in preventing disease progression can be negatively influenced by the high prevalence of alcohol and of illicit substance use among many HIV-infected patients (Montaner et al., 2010; Justice et al., 2006; Wu, Metzger, Lynch, & Douglas, 2011; Kerr et al., 2007; Trenz et al., 2012). Thus, a comprehensive assessment of the relationship between alcohol and illicit substance use on HAART outcomes is warranted.
METHODS
Data
Data from eligible participants were extracted from the British Columbia (BC) Centre for Excellence in HIV/AIDS’ Drug Treatment Program (BCCfE) and from the study “Longitudinal Investigations into Supportive and Ancillary Health Services” (LISA) (Duncan et al., 2012). We included individuals from the LISA study who have initiated HAART for the first time from August, 1996 and until January, 2010. More details regarding the study data can be found in the Supplemental Material. In BC, the HIV epidemic is concentrated among injection drug users and men who have sex with men, and LISA participants reflect those infected with HIV via injection drug use. The final sample size was 1000, however, data from 83 participants excluded since they could not be linked to our clinical data.
Laboratory Monitoring
The BCCfE distributes antiretrovirals, at no cost, to all eligible individuals in BC; and it maintains HIV-specific monitoring clinical data on all individuals who have ever received HAART in BC. Thus, CD4 cell count and viral load data at the start of HAART and at the time of the interview were obtained for our study sample. Viral load was measured using different assays over time. Because the change in viral load assays, the lower and upper limits of quantification of these assays changed over time, and therefore, viral load measurements were re-coded to range from 500 to 100,000 copies/mL to minimize the measurement bias that would have been introduced if the data were left unchanged.
CAGE Questionnaire
CAGE is a commonly used screening tool to detect alcohol dependence (Chander, 2011; Mayfield, McLeod, & Hall, 1974; Ewing, 1984). It has been applied in the general medical population in primary care settings and among HIV-positive populations (Ewing, 1984; Samet, Phillips, Horton, Traphagen, & Fredberg, 2004). It contains four questions: Domain 1 (Cut): Have you ever felt you should cut down on your drinking? (yes/no); Domain 2 (Annoyed): Have people annoyed you by criticizing your drinking? (yes/no); Domain 3 (Guilt): Have you ever felt bad or guilty about your drinking? (yes/no); and Domain 4 (Eye Opener): Have you ever had a drink first thing in the morning (as an “eye opener”) to steady your nerves or get rid of a hangover? (yes/no). In this study, we categorized individuals as having no indication of having a history of alcoholism (i.e. yes to at most 1 question), as having an indication of having a history of alcoholism (i.e. yes to 2 or 3 questions), and as having a confirmed history of alcoholism (i.e. yes to all 4 questions).
Outcome Variables, Main Exposure and Confounder Factors
Our main exposure, substance use, was a three-level categorical variable defined as: no history of alcohol and injection drug use, history of alcohol or injection drug use, and history of both alcohol and injection drug use. Disease progression was defined both virologically and immunologically, as:
By combining each of the levels of viral and CD4 responses, our outcome was a three-level ordinal variable: CD4+/pVL+ (Best); CD4+/pVL− or CD4−/pVL+ (Incomplete); and CD4−/pVL− (Worst).
The potential confounders included baseline age, sex, CD4 cell count, adherence (measured 6 months prior to the LISA interview date), viral load (log10 transformed), baseline HAART regimen type and follow-up time (in years) from baseline to the interview. HAART regimen consisted of two nucleosides, or a nucleoside and a nucleotide reverse transcriptase inhibitor plus either: (1) a non-nucleoside reverse transcriptase inhibitor [NNRTI], or (2) a protease inhibitor boosted with ≤400mg/day ritonavir [boosted PI], or (3) a single protease inhibitor [unboosted PI]. Adherence was defined as the number of days of antiretroviral drugs dispensed divided by the number of days on antiretroviral therapy (expressed as percent). Adherence was categorized as ≥95% (adherent) versus <95% (non-adherent).
Statistical Analysis
In bivariable analyses, categorical variables were compared using the Fisher's exact test, and continuous variables were compared using the Wilcoxon rank sum test. We built a confounder model using cumulative logit modeling for ordinal response (Lima et al., 2012; Lee, 1992; Stokes, Davis, & Koch, 2000). We have adjusted all our multivariable analyses for measurement bias using inverse-probability-weights (Lima et al., 2012), since our main exposure and some of the potential confounders were self-reported. This particular adjustment has been shown to properly handle biases such as measurement bias. All reported p-values are two-sided, and all analyses were performed using SAS version 9.3 (SAS Institute Inc.).
RESULTS
Of the 537 eligible individuals, 141 were female (26%), 392 (73%) had a history of injection drug use, 230 (43%) started on a NNRTI-based regimen and 172 (32%) on a boosted PI regimen. Those with a history of both alcohol and injection drug use were more likely to be female, to have lower CD4 cell count at the time of interview, and a lower baseline viral load (P<0.001). These individuals also experienced a lower CD4 cell count increase from baseline, and a lower viral load change from baseline (P<0.001) (Table 1).
Table 1.
Baseline and interview characteristics associated with the interaction of CAGE questionnaire results and history of injection drug use status for the participants in the LISA cohort.
| Factors | Interaction of CAGE Questionnaire Results and History of Injection Drug Use | |||
|---|---|---|---|---|
| No history of alcohol and injection drug use N = 100 |
History of alcohol or injection drug use N = 325 |
History of both alcohol and injection drug use N = 112 |
p-value | |
| Sex | ||||
| Male | 88 (88%) | 233 (72%) | 75 (67%) | <0.0001 |
| Female | 12 (12%) | 92 (28%) | 37 (33%) | |
| Baseline HAART Regimen | ||||
| Unboosted PI | 26 (26%) | 85 (26%) | 24 (21%) | 0.6554 |
| Boosted PI | 36 (36%) | 101 (31%) | 35 (31%) | |
| NNRTI | 38 (38%) | 139 (43%) | 53 (47%) | |
| Adherence to HAART (6 months before interview) | ||||
| ≥95% | 86 (86%) | 220 (68%) | 58 (52%) | <0.0001 |
| <95% | 14 (14%) | 105 (32%) | 54 (48%) | |
| Baseline Age (years) | 43 (36 – 49) | 40 (33 – 47) | 41 (36 – 45) | 0.1856 |
| CD4 cell count (cells/mm3) | ||||
| Baseline | 225 (80 – 315) | 190 (110 – 290) | 190 (145 – 350) | 0.3000 |
| At the time of interview | 500 (345 – 660) | 350 (210 – 550) | 280 (170 – 430) | <0.0001 |
| • Percent change from baseline | 128 (50 – 393) | 93 (4 – 260) | 30 (−14 – 135) | <0.0001 |
| • Difference from baseline | 260 (140 – 400) | 159 (10 – 320) | 65 (−35; – 220) | <0.0001 |
| Plasma Viral Load (log10 copies/mL) | ||||
| Baseline | 5 (4.8 – 5.0) | 5 (4.6 – 5.0) | 4.8 (4.3 – 5.0) | 0.0001 |
| • Difference from baseline | −2.3 (−2.3 – −2.0) | −2.2 (−2.3 – −1.5) | −1.7 (−2.3 – −0.6) | <0.0001 |
| Follow-up from baseline to the interview (years) | 5.2 (2.0 – 9.2) | 5.3 (2.2 – 8.8) | 3.9 (2.2 – 8.4) | 0.4067 |
NNRTI: non-nucleoside reverse transcriptase inhibitor; Boosted PI: protease inhibitor boosted with ≤400mg/day ritonavir; Unboosted PI: single protease inhibitor.
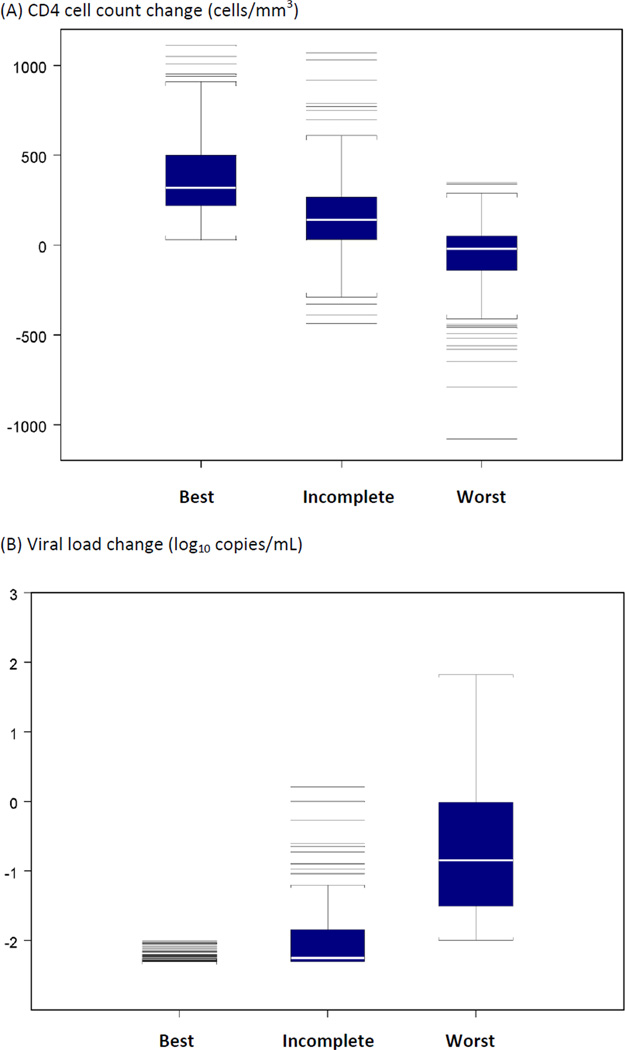
A total of 196 (36%) individuals had CD4+/pVL+ (Best) response, 180 (34%) a CD4+/pVL− or a CD4−/pVL+ (Incomplete) response, and 161 (30%) a CD4−/pVL− (Worst) response (Table 2). Individuals with response CD4−/pVL− (Worst) were more likely to have a confirmed history of alcoholism, to have a history of both alcohol and injection drug use, to be female, to have a history of injection drug use, to have started HAART on an unboosted PI, and to be <95% adherent (P<0.001) (Table 2). Individuals with Worst response, in comparison to Best or Incomplete responses, were more likely to be younger, to have a lower CD4 cell count at the time of the interview, a lower CD4 cell count increase from baseline, a lower baseline viral load and a lower viral load change from baseline (P<0.0001) (Table 2 and Figure 1). Based on the multivariable confounder model, the estimated probabilities of Best, Incomplete and Worse responses were, respectively, (0.51, 0.29, 0.20) for individuals with no history of alcohol and injection drug use, (0.34, 0.32, 0.33) for individuals with a history of alcohol or injection drug use, and (0.15, 0.25, 0.60) for individuals with a history of both alcohol and injection drug use (Table 3).
Table 2.
Baseline and interview characteristics associated with disease progression for the participants in the LISA cohort.
| Factors | CD4+/pVL+ (Best) N = 196 |
CD4+ /pVL− or CD4−/pVL+ (Incomplete) N = 180 |
CD4− /pVL− (Worst) N = 161 |
p-value |
|---|---|---|---|---|
| CAGE Questionnaire Results | ||||
| No indication of having a history of alcoholism | 107 (55%) | 95 (53%) | 50 (31%) | <0.0001 |
| Indication of having a history of alcoholism | 63 (32%) | 53 (29%) | 47 (29%) | |
| Confirmed history of alcoholism | 26 (13%) | 32 (18%) | 64 (40%) | |
| CAGE & History of injection drug use | ||||
| No history of alcohol and injection drug use | 48 (24%) | 38 (21%) | 14 (9%) | <0.0001 |
| History of alcohol or injection drug use | 125 (64%) | 114 (63%) | 86 (53%) | |
| History of both alcohol and injection drug use | 23 (12%) | 28 (16%) | 61 (38%) | |
| Sex | ||||
| Male | 164 (84%) | 132 (73%) | 100 (62%) | <.0001 |
| Female | 32 (16%) | 48 (27%) | 61 (38%) | |
| History of injection drug use | ||||
| No | 68 (35%) | 56 (31%) | 21 (13%) | <.0001 |
| Yes | 128 (65%) | 124 (69%) | 140 (87%) | |
| Baseline HAART regimen | ||||
| Unboosted PI | 39 (20%) | 49 (27%) | 47 (29%) | 0.0022 |
| Boosted PI | 83 (42%) | 52 (29%) | 37 (23%) | |
| NNRTI | 74 (38%) | 79 (44%) | 77 (48%) | |
| Adherence to HAART (6 months before interview) | ||||
| ≥95% | 159 (81%) | 142 (79%) | 63 (39%) | <0.0001 |
| <95% | 37 (19%) | 38 (21%) | 98 (61%) | |
| Baseline age (years) | 42 (36 – 48) | 42 (34 – 48) | 38 (32 – 43) | <0.0001 |
| CD4 cell count (cells/mm3) | ||||
| Baseline | 120 (40 – 180) | 240 (150 – 360) | 260 (180 – 420) | <0.0001 |
| At the time of interview | 435 (315 – 670) | 400 (260 – 575) | 240 (140 – 350) | <0.0001 |
| • Percent change from baseline | 291 (150 – 650) | 58 (10 – 138) | −12 (−43 – 26) | <0.0001 |
| • Difference from baseline | 320 (220 – 500) | 140 (30 – 265) | −20 (−140 – 50) | <0.0001 |
| Plasma Viral Load (log 10 copies/mL) | ||||
| Baseline | 5.0 (5.0 – 5.0) | 5.0 (4.7 – 5.0) | 4.4 (4.0 – 4.7) | <0.0001 |
| • Difference from baseline | −2.3 (−2.3 – −2.3) | −2.2 (−2.3 – −1.8) | −0.8 (−1.5 – 0) | <0.0001 |
| Follow-up from baseline to the interview (year) | 4.3 (2.2 – 7.3) | 5.1 (1.9 – 8.9) | 6.5 (2.5 – 6.1) | 0.0609 |
NNRTI: non-nucleoside reverse transcriptase inhibitor; Boosted PI: protease inhibitor boosted with ≤400mg/day ritonavir; Unboosted PI: single protease inhibitor; pVL: Viral load.
Figure 1.
CD4 cell count and viral load change from baseline to the time of the LISA interview by disease progression categories (CD4+/pVL+ (Best); CD4+/pVL− or CD4−/pVL+ (Incomplete); and CD4−/pVL− (Worst).
Table 3.
Estimated probability based on the multivariable confounder models stratified by the interaction of CAGE questionnaire results and history of injection drug use status, using disease progression (CD4+/pVL+ (Best); CD4+/pVL− or CD4−/pVL+ (Incomplete); and CD4−/pVL− (Worst)) as the main outcome.
| Main Variable | Estimated probability | ||
|---|---|---|---|
| CD4+/pVL+ (Best) | CD4+/pVL− or CD4−/pVL+ (Incomplete) |
CD4−/pVL− (Worst) |
|
| CAGE & History of injection drug use | |||
| No history of alcohol and injection drug use | 0.5123 | 0.2892 | 0.1985 |
| History of alcohol or injection drug use | 0.3434 | 0.3244 | 0.3322 |
| History of both alcohol and injection drug use | 0.1472 | 0.2516 | 0.6012 |
DISCUSSION
This study demonstrated that individuals with a history of both alcohol and injection drug use have a higher likelihood of experiencing the worst immunologic and virologic responses, mostly due to poor adherence. Our results are in agreement with other, albeit few, studies looking at the association of alcohol use and injection drug use with HAART adherence and outcomes (Henrich, Lauder, Desai, Sofair, 2008; Michel et al., 2010; Lucas, Gebo, Chaisson, & Moore, 2002). This finding further emphasizes the problem of non-adherence among this population, which may be the result of individuals engaging in high-risk activities, of poverty, of improper housing and nutrition (Palepu, Milloy, Kerr, Zhang, & Wood, 2011; Weiser et al., 2009; Milloy, Marshall, Montaner, & Wood, 2012; Anema et al., 2011).
This study has some limitations. Because of the LISA study’s design, certain marginalized populations were over sampled and, therefore, our results are not representative of all HIV-infected individuals in BC. It is also important to mention that studies to date have used different methodologies to measure alcohol use (Chander, 2011). Here, we chose to use the CAGE questionnaire, which assesses problems arising from alcohol use and it does not provide a direct measure of the frequency of alcohol consumption (e.g., short-term binges). Furthermore, we used history of injection drug use instead of ongoing injection drug use, and therefore, this study did not capture changes in drug preference, and it also did not distinguish between opioid and stimulant use. Finally, as in all observational studies, even after adjusting for several demographic and clinical characteristics, unmeasured confounders and other types of biases (e.g. recall and volunteer biases) may have played a role in our results, and for this reason, our findings should be interpreted cautiously.
In conclusion, this study has provided more insight on the negative consequences of having a history of alcohol and injection drug use on treatment outcomes for HIV-positive individuals. It is important, therefore, that before and after starting antiretroviral treatment, physicians screen these individuals for any substance use and extensively discuss their addiction treatment options. Ultimately, the screening and detection of alcohol, injection drug use or polysubstance dependence will identify individuals at high-risk for non-adherence and ideally prevent their HIV disease from progressing to advanced stages where HIV disease can become difficult to manage.
Acknowledgments
Dr. Montaner has received educational grants from, served as an ad hoc advisor to or spoken at various events sponsored by Abbott Laboratories, Agouron Pharmaceuticals Inc., Boehringer Ingelheim Pharmaceuticals Inc., Borean Pharma AS, Bristol-Myers Squibb, DuPont Pharma, Gilead Sciences, GlaxoSmithKline, Hoffmann-La Roche, Immune Response Corporation, Incyte, Janssen-Ortho Inc., Kucera Pharmaceutical Company, Merck Frosst Laboratories, Pfizer Canada Inc., Sanofi Pasteur, Shire Biochem Inc., Tibotec Pharmaceuticals Ltd., and Trimeris Inc. Dr. Hogg has held grant funding in the last 5 years from Merck.
Role of the Sponsors: The funding sources had no role in the choice of methods, the contents or form of this work, or the decision to submit the results for publication.
Funding: This study was supported by capacity building and knowledge transfer grants from the Canadian Institutes of Health Research. Dr. Lima was supported by a grant from the US National Institute on Drug Abuse [R03 DA033851-01] and by a Scholar Award from the Michael Institute for Health Research. Dr. Montaner is supported by the Ministry of Health Services, Province of British Columbia; through a Knowledge Translation Award from the Canadian Institutes of Health Research and through an Avant-Garde Award [1DP1DA026182-01] from the National Institute on Drug Abuse, at the US National Institutes of Health. Dr. Thomas Kerr has received grants from Canadian Institutes of Health Research [MOP-81171, HHP-67262, CIHR-251559, MOP-111039], US National Institutes of Health [R01 DA011591], and Foundation Open Society Institute [OSI-20030805]. Dr. Kerr has also received support from Michael Smith Foundation for Health Research. Dr. Evan Wood has received grants from Canadian Institutes of Health Research [MOP-102742, PHE-104125], and US National Institutes of Health (NIH) [R01 DA028532]. Dr. Wood has also received support from Canada Research Chair program through a Tier 1 Canada Research Chair in Inner City Medicine. Dr. Hogg has held grant funding in the last 5 years from the US National Institutes of Health, Medical Research Council UK, and the Canadian Institutes of Health Research.
Footnotes
Ethical approval: The Centre's HIV/AIDS Drug Treatment program has received ethical approval from the University of British Columbia Ethics Review Committee at its St. Paul's Hospital site. The program also conforms with the province's Freedom of Information and Protection of Privacy Act. Ethical approval for the LISA study was obtained from the University of British Columbia/Providence Health Care, Vancouver Coastal Health, University of Victoria and Simon Fraser University Research Ethics Boards.
Author Contributions: Study concept and design: VDL, TK, EW, TK, RH, KS, JSGM; Acquisition of data: JSGM, RH; Analysis and interpretation of data: VDL; Drafting of the manuscript: VDL; Critical revision of the manuscript for important intellectual content and final approval of the version to be submitted to the journal: VDL, TK, EW, TK, RH, KS, JSGM; Statistical analysis: VDL; Obtained funding: VDL, RH; Administrative, technical, or material support: JSGM.
Financial Disclosures: We have the following conflicts of interest:
The remaining authors do not have conflicts to declare.
REFERENCES
- Anema A, Weiser SD, Fernandes KA, Ding E, Brandson EK, Palmer A, Hogg RS. High prevalence of food insecurity among HIV-infected individuals receiving HAART in a resource-rich setting. AIDS Care. 2011;2:221–230. doi: 10.1080/09540121.2010.498908. [DOI] [PubMed] [Google Scholar]
- Chander G. Addressing alcohol use in HIV-infected persons. Topics in Antiviral Medicine. 2011;19(4):143–147. [PMC free article] [PubMed] [Google Scholar]
- Duncan KC, Salters K, Forrest JI, Palmer AK, Wang H, O'Brien N, Hogg RS. Cohort Profile: Longitudinal Investigations into Supportive and Ancillary health services. International Journal of Epidemiology. 2012 doi: 10.1093/ije/dys035. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing JA. Detecting alcoholism. The CAGE questionnaire. The Journal of the American Medical Association. 1984;252(14):1905–1907. doi: 10.1001/jama.252.14.1905. [DOI] [PubMed] [Google Scholar]
- Henrich TJ, Lauder N, Desai MM, Sofair AN. Association of alcohol abuse and injection drug use with immunologic and virologic responses to HAART in HIV-positive patients from urban community health clinics. Journal of Community Health. 2008;33(2):69–77. doi: 10.1007/s10900-007-9069-1. [DOI] [PubMed] [Google Scholar]
- Justice AC, Lasky E, McGinnis KA, Skanderson M, Conigliaro J, Fultz SL VACS 3 Project Team. Medical disease and alcohol use among veterans with human immunodeficiency infection: A comparison of disease measurement strategies. Medical care. 2006;44(8 Suppl 2):S52–S60. doi: 10.1097/01.mlr.0000228003.08925.8c. [DOI] [PubMed] [Google Scholar]
- Kerr T, Fairbairn N, Tyndall M, Marsh D, Li K, Montaner J, Wood E. Predictors of nonfatal overdose among a cohort of polysubstance-using injection drug users. Drug and Alcohol Dependence. 2007;87(1):39–45. doi: 10.1016/j.drugalcdep.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Lee J. Cumulative logit modelling for ordinal response variables: applications to biomedical research. Computer Applications in the Biosciences. 1992;8(6):555–562. doi: 10.1093/bioinformatics/8.6.555. [DOI] [PubMed] [Google Scholar]
- Lima VD, Nosyk B, Wood E, Kozai T, Zhang W, Chan K, Montaner JS. Assessing the effectiveness of antiretroviral regimens in cohort studies involving HIV-positive injection drug users. AIDS. 2012;26(12):1491–1500. doi: 10.1097/QAD.0b013e3283550b68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas GM, Gebo KA, Chaisson RE, Moore RD. Longitudinal assessment of the effects of drug and alcohol abuse on HIV-1 treatment outcomes in an urban clinic. AIDS. 2012;16(5):767–774. doi: 10.1097/00002030-200203290-00012. [DOI] [PubMed] [Google Scholar]
- Mayfield D, McLeod G, Hall P. The CAGE questionnaire: validation of a new alcoholism screening instrument. The American Journal of Psyhiatry. 1974;131(10):1121–1123. doi: 10.1176/ajp.131.10.1121. [DOI] [PubMed] [Google Scholar]
- Michel L, Carrieri MP, Fugon L, Roux P, Aubin HJ, Lert F VESPA Study Group. Harmful alcohol consumption and patterns of substance use in HIV-infected patients receiving antiretrovirals (ANRS-EN12-VESPA Study): relevance for clinical management and intervention. AIDS Care. 2010;22(9):1136–1145. doi: 10.1080/09540121003605039. [DOI] [PubMed] [Google Scholar]
- Milloy MJ, Marshall BD, Montaner J, Wood E. Housing Status and the Health of People Living with HIV/AIDS. Current HIV/AIDS Reports. 2012;9(4):364–374. doi: 10.1007/s11904-012-0137-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaner JS, Lima VD, Barrios R, Yip B, Wood E, Kerr T, Kendall P. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet. 2010;376(9740):532–539. doi: 10.1016/S0140-6736(10)60936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palepu A, Milloy MJ, Kerr T, Zhang R, Wood E. Homelessness and adherence to antiretroviral therapy among a cohort of HIV-infected injection drug users. Journal of Urban Health. 2011;88(3):545–555. doi: 10.1007/s11524-011-9562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samet JH, Phillips SJ, Horton NJ, Traphagen ET, Freedberg KA. Detecting alcohol problems in HIV-infected patients: use of the CAGE questionnaire. AIDS Research and Human Retroviruses. 2004;20(2):151–155. doi: 10.1089/088922204773004860. [DOI] [PubMed] [Google Scholar]
- Stokes ME, Davis CS, Koch GG. Categorical Data Analysis Using the SAS System. 2nd ed. Cary, NC: SAS Institute Inc.; 2000. [Google Scholar]
- Thompson MA, Aberg JA, Hoy JF, Telenti A, Benson C, Cahn P, Volberding PA. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society-USA panel. The Journal of the American Medical Association. 2012;308(4):387–402. doi: 10.1001/jama.2012.7961. [DOI] [PubMed] [Google Scholar]
- Trenz RC, Scherer M, Harrell P, Zur J, Sinha A, Latimer W. Early onset of drug and polysubstance use as predictors of injection drug use among adult drug users. Addictive Behaviours. 2012;37(4):367–372. doi: 10.1016/j.addbeh.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser SD, Fernandes KA, Brandson EK, Lima VD, Anema A, Bangsberg DR, Hogg RS. The association between food insecurity and mortality among HIV-infected individuals on HAART. Journal of Acquired Immune Deficiency Syndromes. 2009;52(3):342–349. doi: 10.1097/QAI.0b013e3181b627c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu ES, Metzger DS, Lynch KG, Douglas SD. Association between alcohol use and HIV viral load. Journal of Acquired Immune Deficiency Syndromes. 2011;56(5):e129–e130. doi: 10.1097/QAI.0b013e31820dc1c8. [DOI] [PMC free article] [PubMed] [Google Scholar]