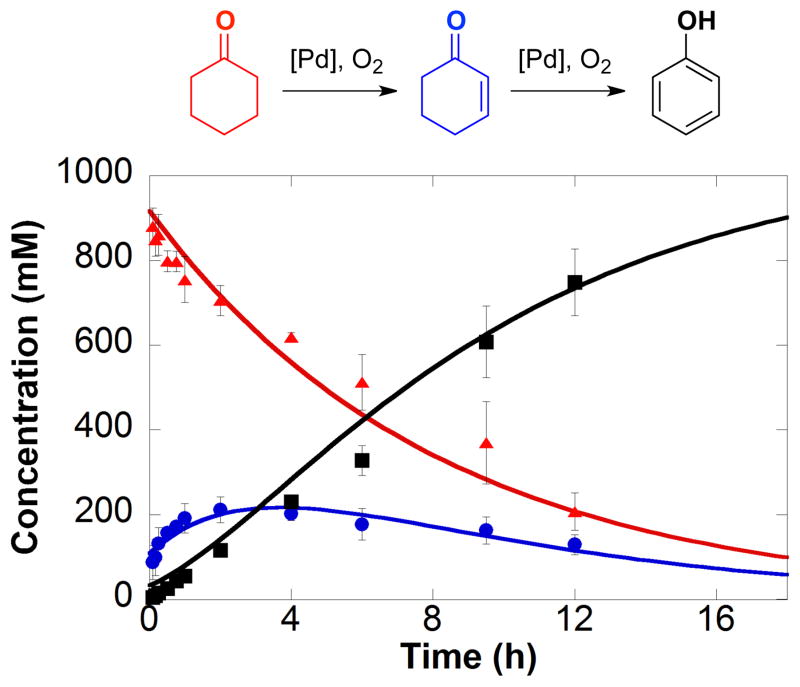
Figure 1.
Kinetic time course of the dehydrogenation of cyclohexanone (
 ) to phenol (■), with cyclohexenone (
) to phenol (■), with cyclohexenone (
 ) observed as the intermediate. Reaction conditions: [cyclohexanone] = 1.0 M (0.5 mmol), [Pd(TFA)2] = 0.05 M (0.025 mmol), [2-Me2Npy] = 0.1 M (0.05 mmol), [TsOH] = 0.2 M (0.1 mmol), DMSO for total volume of 0.5 mL, 1 atm O2, 80 °C. Figure adapted from reference 3a.
) observed as the intermediate. Reaction conditions: [cyclohexanone] = 1.0 M (0.5 mmol), [Pd(TFA)2] = 0.05 M (0.025 mmol), [2-Me2Npy] = 0.1 M (0.05 mmol), [TsOH] = 0.2 M (0.1 mmol), DMSO for total volume of 0.5 mL, 1 atm O2, 80 °C. Figure adapted from reference 3a.