Abstract
(S)-2-Hydroxypropylphosphonic acid ((S)-2-HPP) epoxidase (HppE) is an unusual mononuclear non-heme iron enzyme that catalyzes the oxidative epoxidation of (S)-2-HPP in the biosynthesis of the antibiotic fosfomycin. Recently, HppE has been shown to accept (R)-1-hydroxypropylphosphonic acid ((R)-1-HPP) as a substrate and convert it to an aldehyde product in a reaction involving a biologically unprecedented 1,2-phosphono migration. In this study, a series of substrate analogues were designed, synthesized, and used as mechanistic probes to study this novel enzymatic transformation. The resulting data, together with insights obtained from density functional theory calculations, are consistent with a mechanism of HppE-catalyzed phosphono group migration that involves the formation of a carbocation intermediate. As such, this reaction represents a new paradigm for biological C-P bond cleavage.
Keywords: epoxidase, fosfomycin, C-P bond cleavage, mechanistic studies, substrate analogues
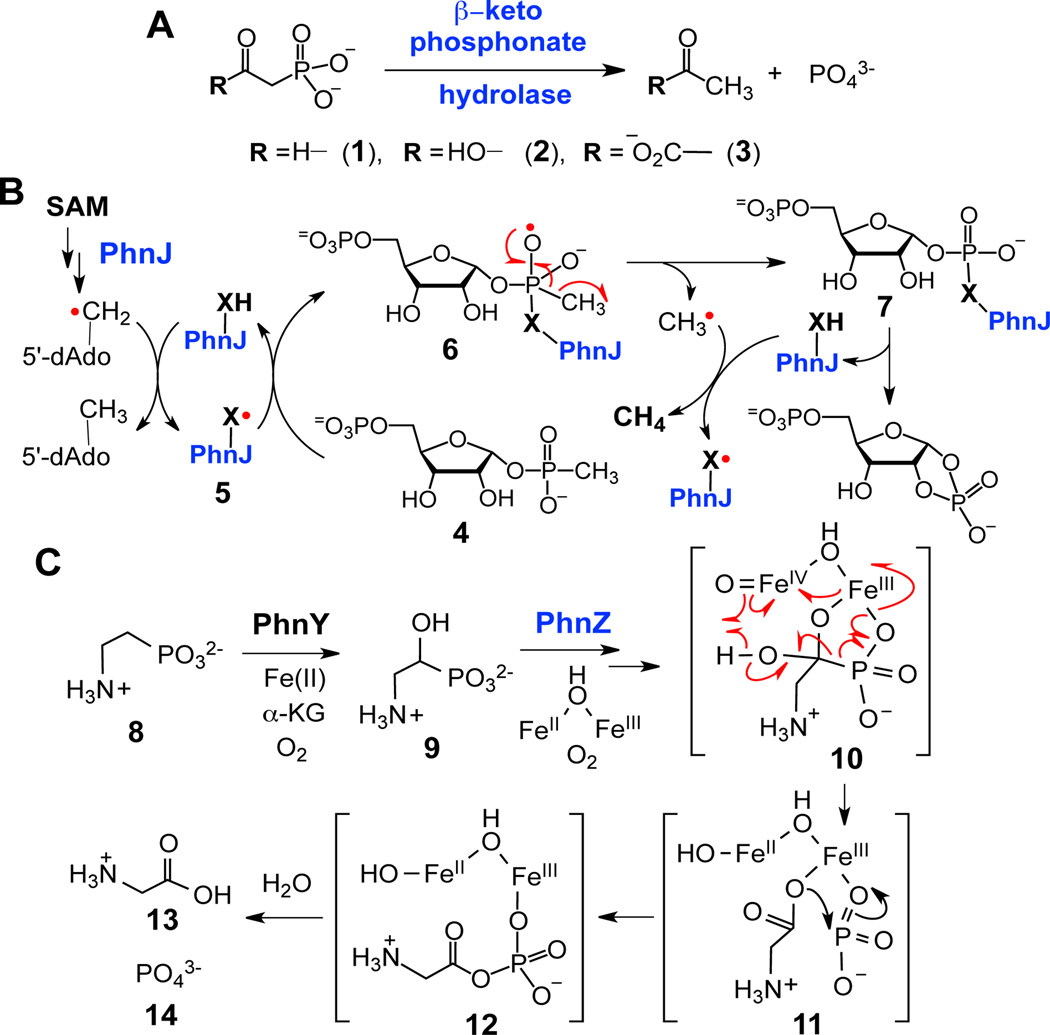
Phosphorus is an essential element for life, where it is found as an integral component of nucleic acids, phospholipids, and many other important biomolecules.1 In biological systems, phosphorus is typically present in the form of inorganic phosphate or derivatives, such as organophosphate esters and anhydrides.1 However, in recent years it has become increasingly apparent that more highly reduced phosphorus compounds also play prominent roles in biology.1–2 Many of these compounds, such as the phosphonic and phosphinic acids, contain stable C-P bonds in place of the labile O-P bonds of the corresponding phosphate esters.2 Several such "C-P compounds" are bioactive natural products of agricultural (e.g., phosphinothricin tripeptide, phosphonothrixin) and medical (e.g., fosfomycin, fosmidomycin) importance.1–2 Still other C-P compounds are metabolized as a source of inorganic phosphate by microorganisms living in phosphate-poor environments.1
Several distinct mechanisms of biological C-P bond cleavage have been identified for the degradation of organophosphonates to liberate phosphate for metabolic use (see Scheme 1).1,3–5 For example, the C-P bond cleavage of β-keto-phosphonate substrates, such as phosphonoacetaldehyde (1), phosphonoacetate (2), and phosphonopyruvate (3), catalyzed by the corresponding hydrolases, proceeds via nucleophilic attack at the phosphorus center by a water molecule or an active site nucleophile. The negative charge developed during turnover in these reactions is stabilized by metal ions or a Schiff base.2,4,5 Another example is illustrated by the bacterial C-P lyase pathway, which utilizes a non-canonical radical S-adenosyl-l-methionine (SAM) enzyme, PhnJ, to cleave the C-P bond of 5-phospho-α-d-ribosyl-1-alkylphosphonate (4).3 The detailed reaction mechanism of PhnJ is not fully understood, although it has been proposed to proceed via attack at the phosphorus center by a thiyl radical (5), leading to homolytic cleavage of the C-P bond through a radical-mediated process (6→7).3 Recently, the genes responsible for an alternative organophosphonate catabolic pathway were identified in marine-derived metagenomic DNA. In this pathway, 2-aminoethylphosphonate (8) is converted to glycine (13) and inorganic phosphate (14) by the combined action of PhnY and PhnZ.6 Specifically, PhnZ, a member of the histidine-aspartate (HD) hydrolase superfamily, is proposed to catalyze the oxidative cleavage of the C-P bond of 2-amino-1-hydroxylethyl-phosphonate (9) through a mechanism reminiscent of that used by the diiron enzyme myo-inositol oxygenase (MIOX).6–8 In this mechanism, the high-valent FeIII-FeIV-oxo intermediate (10) induces fragmentation of the C-P bond of the substrate, leading to a metaphosphate intermediate (11) that recombines with the carboxylate anion of glycine to give 12. Subsequent hydrolysis yields 13 and 14.
Scheme 1.
Representative reactions for enzyme-catalyzed C-P bond cleavage reactions.
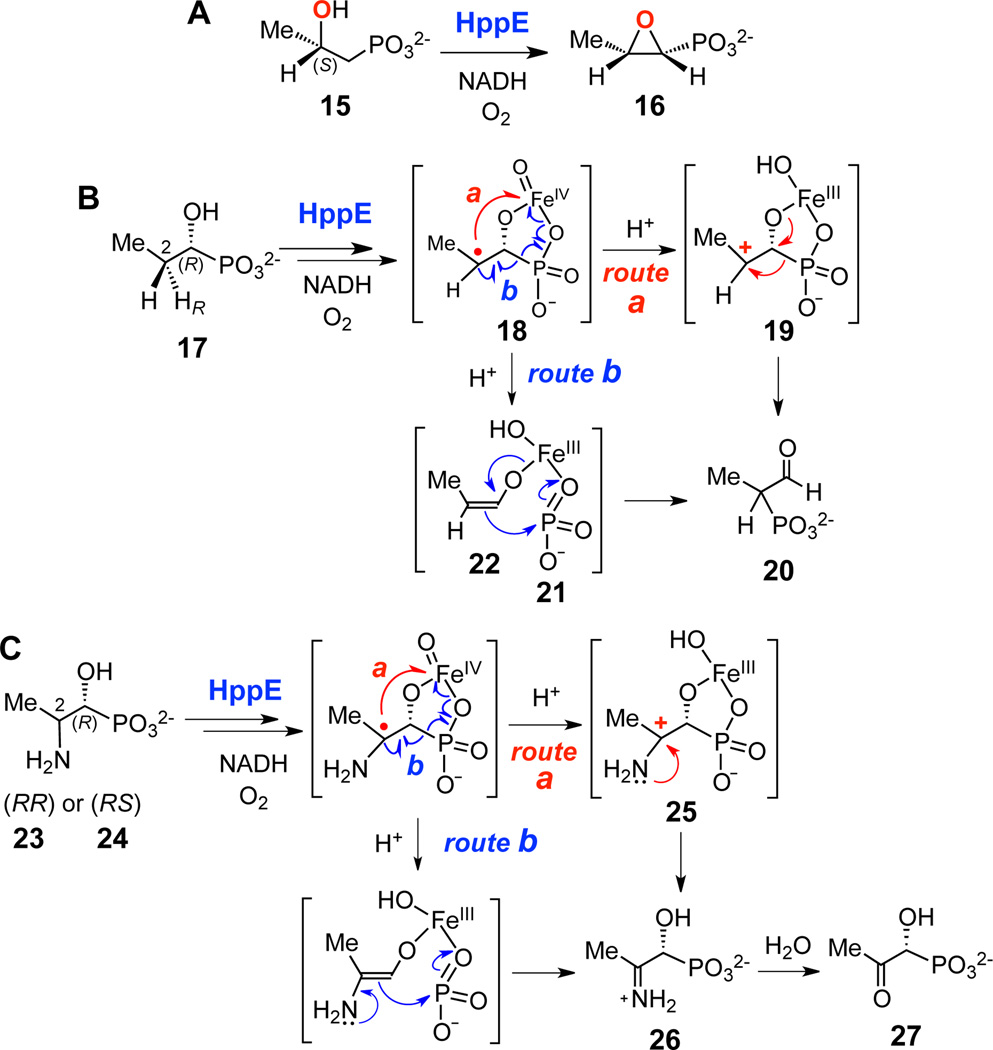
More recently, an oxidative 1,2-phosphono group migration was observed in the reaction of the unusual non-heme iron enzyme (S)-2-hydroxypropylphosphonate (HPP, 15) epoxidase (HppE), which catalyzes the conversion of 15 to fosfomycin (16) (Scheme 2A).9 When incubated with the alternative substrate (R)-1-HPP (17), HppE catalyzes the migration of the phosphonate group of 17 from C1 to C2 to give aldehyde 20 as the product.10 On the basis of results obtained from X-ray crystallography, model chemistry, and reactions with mechanistic probes (e.g., 23 and 24), it was proposed that cleavage of the C-P bond of (R)-1-HPP (17) is induced by the formation of a C2-centered carbocation intermediate (19, Scheme 2B, route a).10 If correct, this would represent a new paradigm for the enzymatic cleavage of a C-P bond and would indicate that such reactions can occur via cation- (Scheme 2B, route a) as well as anion- (Scheme 1A) and radical-mediated (Scheme 1B,C) mechanisms. However, by analogy with the proposed mechanism of PhnZ,6 the HppE-catalyzed C-P bond migration observed with (R)-1-HPP (17) can also be explained by a radical-induced fragmentation to generate metaphosphate (21) and an enolate anion intermediate (22), followed by recombination to give the corresponding products (20, Scheme 2B, route b). Similar mechanisms can also account for the formation of 27 from the corresponding 2-amino analogue 23 and 24 (Scheme 2C, routes a and b). To distinguish between these two fundamentally different mechanisms, additional analogues of (R)-1-HPP bearing electronwithdrawing and/or leaving groups at the C3 position were designed, synthesized, and analyzed as mechanistic probes of the HppE-catalyzed 1,2-phosphono migration reaction.
Scheme 2.
The reaction catalyzed by HppE (A) and the proposed mechanisms to account for the reactions of HppE with (R)-1-HPP (17) (B) and (1R,2R)- or (1R,2S)-2-amino-1-HPP (23 or 24) (C). Route a, carbocation rearrangement. Route b, radical-induced fragmentation/recombination.
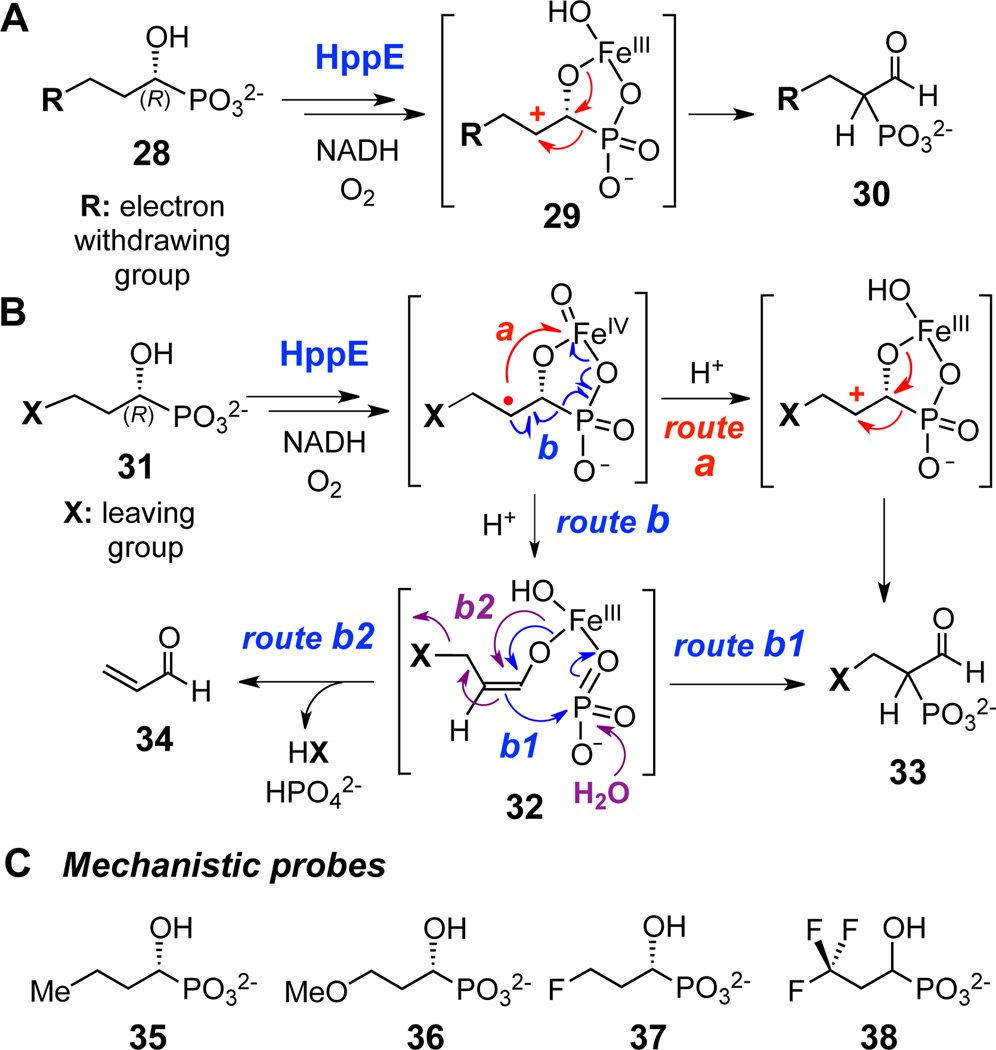
The presence of one or more electron-withdrawing groups on the carbon adjacent to the carbocation center will dramatically affect the stability of the cation.11,12 For example, in studies of prenyltransferase, which catalyzes a reaction generally accepted to proceed with formation of a carbocation intermediate, the reaction rate was decreased ~3 × 107-fold when one of the methyl groups of the substrate was replaced with a trifluoromethyl group.13 Thus, one would expect a dramatic rate reduction of HppE-catalyzed phosphono migration (29→30, Scheme 3A) that reflects the electron-withdrawing property of the C3 substituent of the substrate analogues (R in 28), if the reaction proceeds via the carbocation rearrangement mechanism (Scheme 2B, route a). In contrast, if the radical-induced fragmentation/recombination mechanism is operative (Scheme 2B, route b), analogues containing a leaving group at C3 (e.g., 31) will partition between formation of the 1,2-phosphono migration product (33, Scheme 3B, route b1) and elimination of the leaving group from the enolate anion intermediate (32) with concomitant and subsequent formation of acrylaldehyde (34) and inorganic phosphate, respectively (Scheme 3B, route b2).
Scheme 3.
Possible reaction outcomes of HppE with substrate analogues bearing electron-withdrawing (A) and/or leaving groups at C3 (B). (C) List of mechanistic probes used in this study.
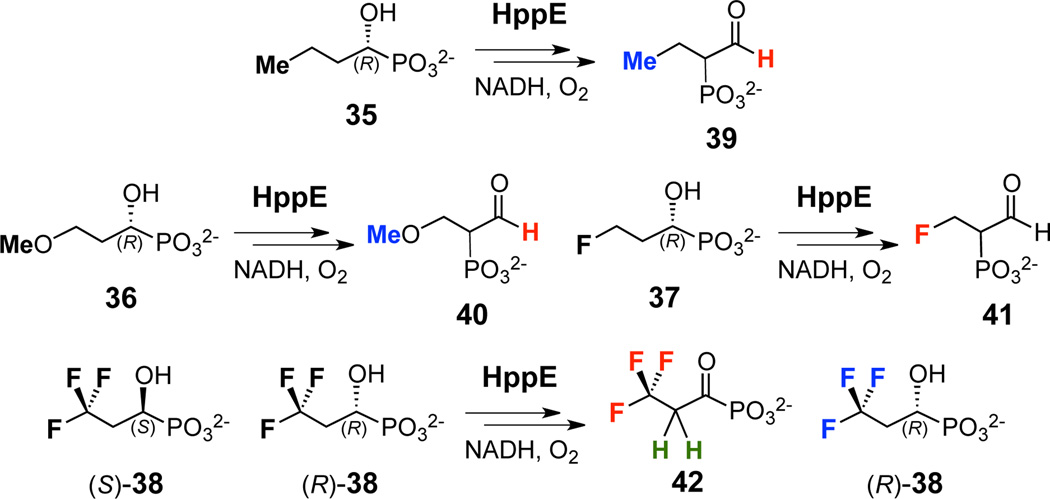
With these scenarios in mind, probes bearing either methoxy (36), monofluoro (37), or trifluoro (38) groups were prepared (Scheme 3C).14 In addition, an analogue with a terminal methyl group (35) was also synthesized.14 The trifluoro group-containing analogue (38) was synthesized as a racemic mixture due to poor reactivity with porcine esterase and the various lipases used to resolve the C1-OH chirality. The remaining analogues were obtained in enantiomerically pure form, as determined by measuring the enantiomeric excess of each compound by 31P NMR using quinine as a chiral shift reagent15 (see Supporting Information).
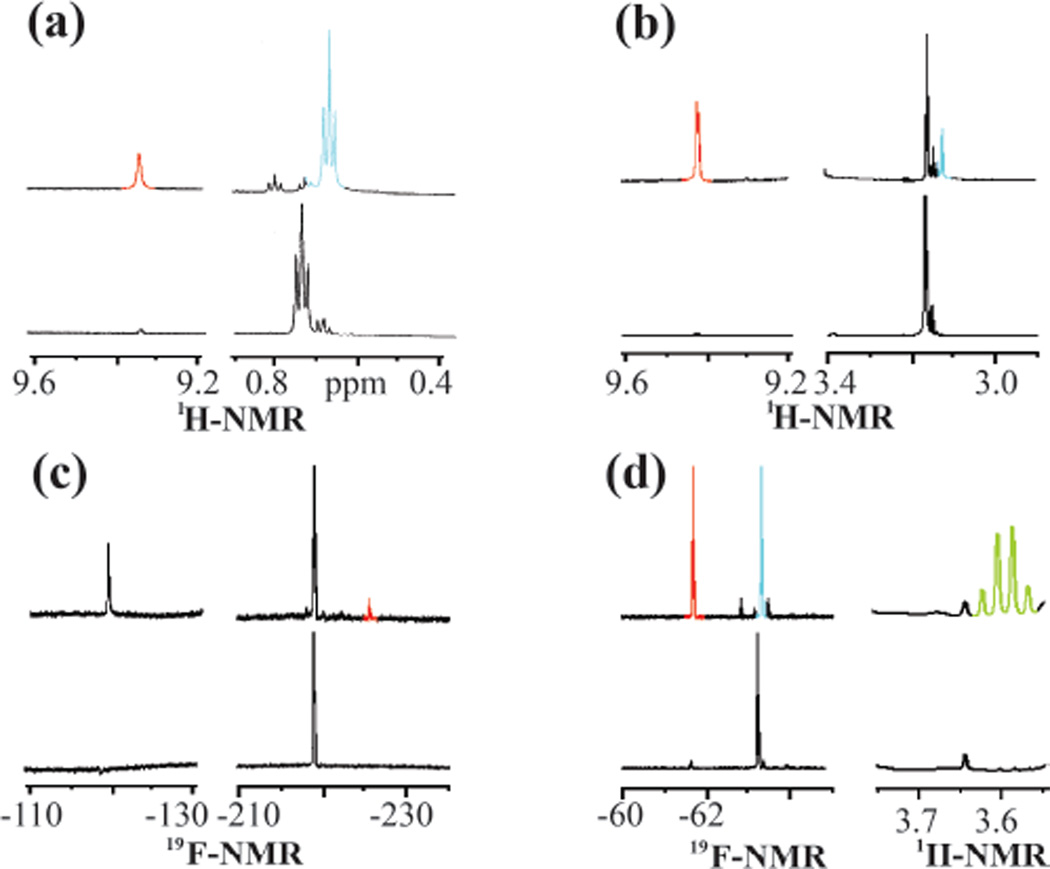
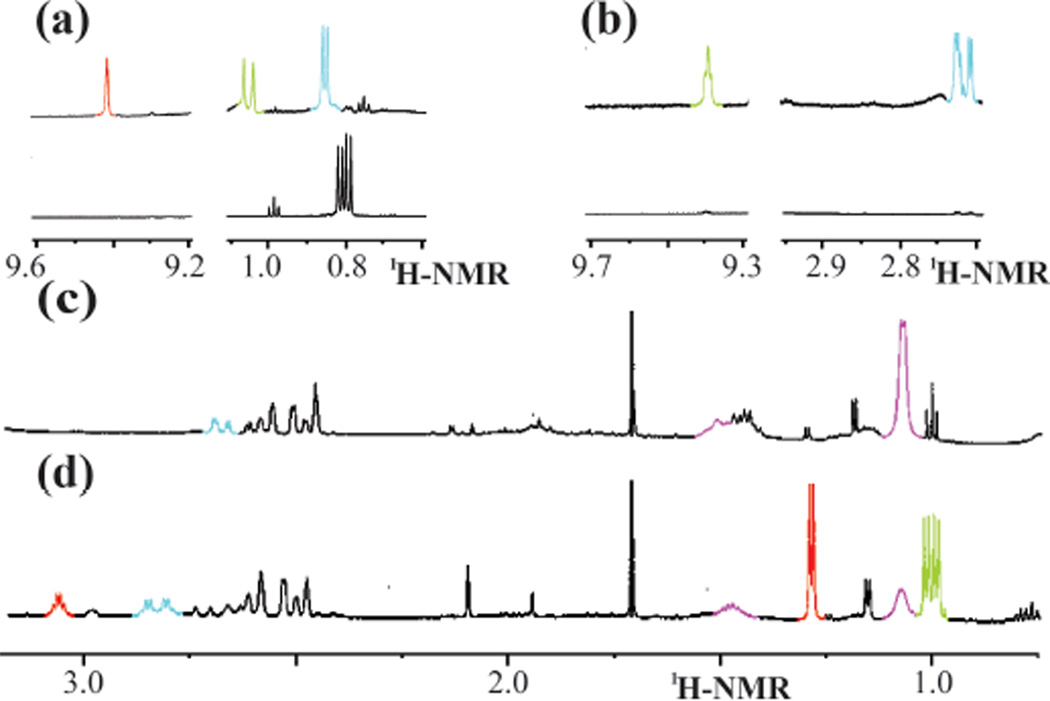
Following the previously reported procedure,10 the reaction of HppE with 35 was monitored using 1H NMR spectroscopy over a 30 min period, and the extent of substrate consumption/product formation at various time points was estimated by peak integration and comparison with an internal standard (DMSO-d6, δ 2.49). A new signal with a chemical shift consistent with an aldehyde proton (δ 9.32) was observed. Its appearance is accompanied by the shift of the methyl resonance from δ 0.75 (of 35) to δ 0.68 (of 39), indicating that HppE can accept 35 as a substrate and convert it nearly quantitatively to the corresponding migration product (39) (Figure 1a and Scheme 4). The results show that HppE can tolerate an increase in the steric bulk of the substrate.
Figure 1.
Selected 1H and 19F NMR assays of HppE with substrate analogues: (a) 35, (b) 36, (c) 37, and (d) 38. The bottom trace of each panel is the spectrum taken 3 min after mixing HppE with the substrate analogue, and the top trace is recorded 27 min after initiation of the reaction. The NMR signals and the corresponding protons in the structures shown in Scheme 4 are colorcoded.
Scheme 4.
Reactions of HppE with substrate analogues 35–38.
When the experiments were repeated under the same conditions but with the 3-methoxy analogue 36 as the substrate, the distinctive aldehyde proton peak of the migration product 40 (δ 9.42) was detected, although the extent of conversion was only 16% during the time monitored (Figure 1b). Neither methoxy group elimination nor acrylaldehyde formation was noted using 1H NMR spectroscopy. When 37 was incubated with HppE under the same conditions, only a trace amount of the migration product (41) was detected by 1H NMR spectroscopy (Scheme 4 and see Figure S1 in the Supporting Information). The conversion was less than 5% based on the integration of the 19F NMR peaks (Figure 1c). Interestingly, in addition to the fluoro resonances of the substrate and product (δ −218 and −225, respectively), a fluoride anion signal at δ −121 was also detected by 19F NMR spectroscopy (Figure 1c). However, this fluoride anion peak remains visible in the absence of enzyme and no inorganic phosphate formation was discernible using 31P NMR spectroscopy. Thus, the appearance of fluoride anion during incubation is unlikely a catalytically relevant event.
When the substrate analogue bearing a trifluoro methyl group at C3 (38) was incubated with HppE, ~50% of 38 was converted to the corresponding acyl phosphonate (42) (estimated based on the integration of the 19F NMR resonances at δ −63.2 of 38 and δ −61.6 of 42, Figure 1d). A quintet (JH-F = 10.8 Hz) at δ 3.60 in the 1H NMR spectrum (Figure 1d) is characteristic for the C2 methylene protons of 42. No obvious formation of the phosphono migration product was discernible. The fact that only half of 38 was consumed during the course of the reaction is not surprising since 38 used in the incubation is a racemic mixture. It appears that only the S-epimer is accepted as a substrate by HppE and is converted to the expected acyl phosphonate product (42), whereas the R-epimer, which should give rise to the migration product, does not react (Scheme 4).10,16 Given the inverse correlation between the extent of conversion of the substrate analogues to the corresponding migration products and the electron-withdrawing ability of the substituents, these results are more consistent with a mechanism involving the formation of a carbocation intermediate along the reaction coordinate.
To further explore the effects of electron-withdrawing substitution with the (R)-1-HPP substrate analogues on the energetics of the HppE-catalyzed reactions, density functional theory (DFT) calculations were performed (see Supporting Information). The calculated C2-H bond dissociation energies (BDEs) for the methoxy-containing substrate analogue 36 (93.9 kcal/mol) and the monofluoro analogue 37 (93.5 kcal/mol) are very similar to that of (R)-1-HPP (93.8 kcal/mol),10 whereas the BDE for the trifluoro analogue (38) is significantly higher (98.4 kcal/mol). The increased strength of the C-H bond of 38 may explain the inability of HppE to accept the R-isomer as a substrate; however, BDEs alone cannot explain the observed differences in yield with analogues 36 and 37 (16% and <5% versus 17, respectively). This prompted an examination of the relative ionization energies (IEs) of each of the corresponding C2-centered radicals of analogues 36–38.17 The IEs of the substrate analogue radicals were found to increase as a function of the electron-withdrawing ability of the C3 substituent, from 4.5 to 15.2 to 26.3 kcal/mol (relative to the IE of the (R)-1-HPP radical) for analogues 36–38, respectively. This increase in IE closely follows the observed trend in reactivity of HppE with the substrate analogues, supporting the involvement of carbocation formation in the 1,2-phosphono migration reaction (Scheme 2B, route a).
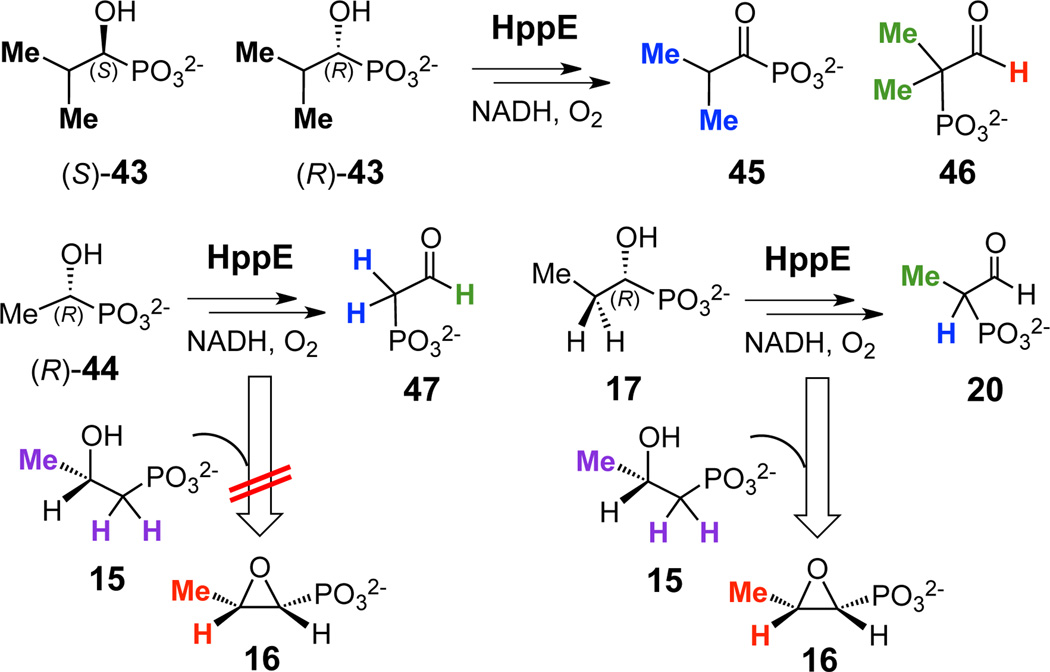
The C2 radicals/cations formed in the reactions of HppE with (R)-1-HPP (17) and analogues 36–38 are centered at a 2° carbon. It is well known that the stability of both radicals and cations increase in the order 1° < 2° < 3°.11,12 Moreover, the stabilization effects are more dramatic for cations than the corresponding radicals.12 As a result, an analogue of (R)-1-HPP proceeding through a 3° carbocation intermediate is expected to be readily converted by HppE to the subsequent migration product, while an analogue that requires the formation of a 1° carbocation is likely to be a poor substrate. Two compounds, 43 and 44, were therefore prepared to test this hypothesis and gain further evidence for the proposed carbocation mechanism.14
Compound 43, which has an isopropyl group attached to C1, was synthesized as a racemic mixture. Upon incubation with HppE, 43 was completely consumed to produce acyl phosphonate 45 (CH3, δ 0.86, doublet, JH-H = 7.2 Hz) and the phosphono migration product 46 (CH3, δ 1.05, doublet, JP-H = 13.8 Hz; aldehyde-H, δ 9.41) in a 1:1 ratio (as determined by integration of the methyl signals of the products, Figure 2a). Unlike compound 43, the R- and S-isomers of 1-hydroxyethylphosphonate (1-HEP, 44) were prepared separately.14 When (S)-44 was incubated with HppE, quantitative turnover to the acyl phosphonate product was observed (see Figure S2 in Supporting Information). However, under the same conditions, <15% of (R)-44 was converted to the migration product (47) (methylene protons, δ 2.72, doublet of doublets, JP-H = 19.8 Hz, JH-H = 4.2 Hz; aldehyde-H, δ 9.40, triplet, JH-H = 4.2 Hz, Figure 2b). Further experiments showed that preincubation of HppE with (R)-44 led to enzyme inactivation, as the resulting enzyme failed to convert (S)-2-HPP (15) to fosfomycin (16) (Figure 2c). In control reactions where HppE was preincubated with (R)-1-HPP (17), the enzyme remained active and capable of catalyzing fosfomycin production from 15 (Figure 2d). These data suggest that HppE is able to abstract a hydrogen atom from C2 of (R)-44 to initiate the reaction, but only a fraction of the radicals generated are oxidized to the corresponding cation and converted to the phosphono migration product 47; the remaining radicals react with the enzyme, rendering it inactive. This interpretation is consistent with the results of DFT calculations on analogues 43 and 44, which show that the C2-H BDE and IE of the 3° analogue 43 is 3.3 and 7.3 kcal/mol less than that for (R)-1-HPP (17), respectively, while those for the 1° analogue 44 are 3.7 and 10.5 kcal/mol greater than 17.
Figure 2.
Selected 1H NMR assays of HppE with substrate analogues: (a) 43, (b) (R)-44, (c) preincubation with (R)-44 followed by the addition of 15, and (d) preincubation with 17 followed by the addition of 15. The bottom trace in panels (a) and (b) is the spectrum taken 3 min after mixing HppE with the substrate analogue, and the top trace is recorded 27 min after initiation of the reaction. The NMR signals and the corresponding protons in the structures shown in Scheme 5 are color-coded.
In summary, these results, coupled with information obtained from previous mechanistic studies,10,18 are consistent with the following mechanism of HppE-catalyzed 1,2-phosphono migration (see Scheme 2B and Figure S3 in Supporting Information). First, (R)-1-HPP (17) binds to the ferrous iron of HppE in a bidentate fashion through its hydroxyl and phosphonate moieties and organizes the iron center to coordinate molecular oxygen. O2 then binds, generating a ferric-superoxo species that abstracts the pro-R hydrogen atom from C2. Proton-coupled electron transfer to the resulting ferric-hydroperoxo species generates a highly reactive ferryl intermediate (18) that oxidizes the C2-centered substrate radical to the corresponding 2° carbocation (19). The carbocation thus formed induces the 1,2-shift of the phosphono moiety to generate the aldehyde product (20). Finally, product release and reduction of the ferric iron back to the ferrous state completes the catalytic cycle. To our knowledge, this is the first example of the enzymatic cleavage of a C-P bond induced by the formation of a carbocation. As such, it represents a new paradigm for biological C-P bond cleavage.
Supplementary Material
Scheme 5.
Reactions of HppE with substrate analogues 43 and 44.
ACKNOWLEDGMENTS
We would like to dedicate this paper to Professors Koji Nakanishi and Christopher Walsh on the occasion of their 88th (the beiju/rice age) and 70th birthdays, respectively. We wish to thank Steve Sorey for his help in acquiring the NMR spectra.
Funding Sources
This work is supported in part by grants from the National Institutes of Health (GM040541) and the Welch Foundation (F-1511).
Footnotes
ASSOCIATED CONTENT
Supporting Information
Details of synthetic methods for compounds 35–38, 43, and 44. Experimental procedures for the reactions of HppE with substrate analogues. Computational methods used to calculate bond dissociation and ionization energies. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.(a) Seto H, Kuzuyama T. Nat. Prod. Rep. 1999;16:589. doi: 10.1039/a809398i. [DOI] [PubMed] [Google Scholar]; (b) White AK, Metcalf WW. Annu. Rev. Microbiol. 2007;61:379. doi: 10.1146/annurev.micro.61.080706.093357. [DOI] [PubMed] [Google Scholar]
- 2.Metcalf WW, van der Donk WA. Annu. Rev. Biochem. 2009;78:65. doi: 10.1146/annurev.biochem.78.091707.100215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamat SS, Williams HJ, Raushel FM. Nature. 2011;480:570. doi: 10.1038/nature10622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quinn JP, Kulakova AN, Cooley NA, McGrath JW. Environ. Microbiol. 2007;9:2392. doi: 10.1111/j.1462-2920.2007.01397.x. [DOI] [PubMed] [Google Scholar]
- 5.Kim J, Dunaway-Mariano D. Biochemistry. 1996;35:4628. doi: 10.1021/bi952944k. [DOI] [PubMed] [Google Scholar]
- 6.McSorley FR, Wyatt PB, Martinez A, Delong EF, Hove-Jensen B, Zechel DL. J. Am. Chem. Soc. 2012;134:8364. doi: 10.1021/ja302072f. [DOI] [PubMed] [Google Scholar]
- 7.Xing G, Barr EW, Diao Y, Hoffart LM, Prabhu KS, Arner RJ, Reddy CC, Krebs C, Bollinger JM., Jr Biochemistry. 2006;45:5402. doi: 10.1021/bi0526276. [DOI] [PubMed] [Google Scholar]
- 8.Xing G, Diao Y, Hoffart LM, Barr EW, Prabhu KS, Arner RJ, Reddy CC, Krebs C, Bollinger JM., Jr Proc. Natl. Acad. Sci. USA. 2006;103:6130. doi: 10.1073/pnas.0508473103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Liu P, Murakami K, Seki T, He X, Yeung SM, Kuzuyama T, Seto H, Liu H-w. J. Am. Chem. Soc. 2001;123:4619. doi: 10.1021/ja004153y. [DOI] [PubMed] [Google Scholar]; (b) Zhao Z, Liu P, Murakami K, Kuzuyama T, Seto H, Liu H-w. Angew. Chem. Int. Ed. 2002;41:4529. doi: 10.1002/1521-3773(20021202)41:23<4529::AID-ANIE4529>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]; (c) Liu P, Liu A, Yan F, Wolfe MD, Lipscomb JD, Liu H-w. Biochemistry. 2003;42:11577. doi: 10.1021/bi030140w. [DOI] [PubMed] [Google Scholar]; (d) Higgins LJ, Yan F, Liu P, Liu H-w, Drennan CL. Nature. 2005;437:838. doi: 10.1038/nature03924. [DOI] [PubMed] [Google Scholar]
- 10.Chang W-c, Dey M, Liu P, Mansoorabadi SO, Moon S-J, Zhao KZ, Drennan CL, Liu H-w. Nature. 2013;496:114. doi: 10.1038/nature11998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blanksby SJ, Ellison GB. Acc. Chem. Res. 2003;36:255. doi: 10.1021/ar020230d. [DOI] [PubMed] [Google Scholar]
- 12.Kim CK, Lee KA, Bae SY, Han IS, Kim CK. Bull. Korean Chem. Soc. 2004;25:311. [Google Scholar]
- 13.(a) Poulter CD, Rilling HC. Biochemistry. 1976;15:1079. doi: 10.1021/bi00650a019. [DOI] [PubMed] [Google Scholar]; (b) Poulter CD, Satterwhite DM. Biochemistry. 1977;16:5470. doi: 10.1021/bi00644a012. [DOI] [PubMed] [Google Scholar]
- 14.See supporting information for experimental details.
- 15.Żymańczyk-Duda E, Skwarczyński M, Lejczak B, Kafarski P. Tetrahedron-Asymmetry. 1996;7:1277. [Google Scholar]
- 16.In reactions catalyzed by HppE, the configuration of the substrate governs its enzymatic fate.9b The S-epimer of 1-HPP is converted to an acyl phosphonate, whereas the phosphonate migration reaction (and aldehyde formation) is observed with the R-epimer.10
- 17.The calculated IEs were obtained as the difference in the electronic energies of the radical and cationic species using the optimized structure of the corresponding radical. This was done because the phosphonate group was found to migrate to the 2 position during geometry optimization of the cationic species. The reported values can thus be considered an upper limit for the IE differences.
- 18.(a) Yan F, Moon SJ, Liu P, Zhao Z, Lipscomb JD, Liu A, Liu H-w. Biochemistry. 2007;46:12628. doi: 10.1021/bi701370e. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Mirica LM, McCusker KP, Munos JW, Liu H-w, Klinman JP. J. Am. Chem. Soc. 2008;130:8122. doi: 10.1021/ja800265s. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Yun D, Dey M, Higgins LJ, Yan F, Liu H-w, Drennan CL. J. Am. Chem. Soc. 2011;133:11262. doi: 10.1021/ja2025728. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Huang H, Chang W-c, Pai P-J, Romo A, Mansoorabadi SO, Russell DH, Liu H-w. J. Am. Chem. Soc. 2012;134:16171. doi: 10.1021/ja3078126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.