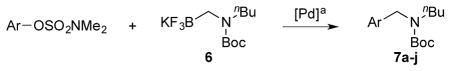
Table 3.
Cross-Coupling of Various Aryl Sulfamates with Secondary Aminomethyltrifluoroborates 6
| |
|---|---|
| entry | product yield(%)b/conversion(%)c |
| 1 |
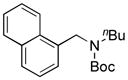 7a, 92/100 |
| 2 |
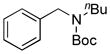 7b, 33/76 |
| 3 |
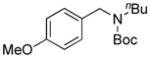 7c, 15b/16 |
| 4 |
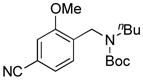 7d, 63/100 |
| 5d |
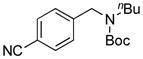 7e, 53/80 |
| 6 |
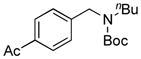 7f, 42/100 |
| 7 |
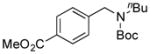 7g, 76/100 |
| 8 |
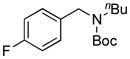 7h, 47/68 |
| 9 |
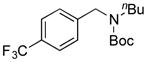 7i, 42/60 |
| 10 |
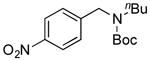 7j, 0/0 |
Reaction conditions: 1.0 equiv of aryl sulfamate, 1.05 equiv of trifluoroborate, 4 mol % XPhos-Pd-G2, 7 equiv of K2CO3, t-BuOH/H2O (1:1, 0.5 M), 85 °C, 18 h.
isolated yield.
calculated by 1H NMR with 30 μL of CH2Cl2.
5 equiv of K2CO3.
