Abstract
Epithelial-mesenchymal transition is a change of cellular plasticity critical for embryonic development and tumor metastasis. CDK5 is a proline-directed serine/threonine kinase playing important roles in cancer progression. Here we show that CDK5 is commonly overexpressed and significantly correlated with several poor prognostic parameters of breast cancer. We found that CDK5 participated in TGF-β1-induced EMT. In MCF10A, TGF-β1 upregulated the CDK5 and p35 expression, and CDK5 knockdown inhibited TGF-β1-induced EMT. CDK5 overexpression also exhibited a potential synergy in promoting TGF-β1-induced EMT. In mesenchymal breast cancer cells MDA-MB-231 and BT549, CDK5 knockdown suppressed cell motility and tumorigenesis. We further demonstrated that CDK5 modulated cancer cell migration and tumor formation by regulating the phosphorylation of FAK at Ser-732. Therefore, CDK5-FAK pathway, as a downstream step of TGF-β1 signaling, is essential for EMT and motility in breast cancer cells. This study implicates the potential value of CDK5 as a molecular marker for breast cancer.
Epithelial-mesenchymal transition (EMT) has been identified as a crucial process in embryonic development. During gastrulation, EMT enables the development of mesoderm from epithelium. EMT also plays very important roles in formation of endocardial cushions of the atrioventricular canal and palate fusion during the development of heart. Generally, EMT is an essential cellular differentiation process that affects tissues as a coordinated unit in the embryogenesis and organogenesis1.
The potential role of EMT in pathological processes has been extensively studied over the years, including its role in the progression of carcinoma and fibrosis of tissues and organs2,3. Oncogenic EMT refers to the process in which epithelial malignant cells acquire mesenchymal cell phenotype, including enhanced migratory capacity and invasiveness, and is generally accepted as a mechanism underlying metastasis in many types of cancer4. Frequently, oncogenic EMT occurs in combination with other anomalies intrinsic to malignant cells, such as the ability to resist to apoptosis and anoikis4,5.
The transforming growth factor-β (TGF-β) has emerged as a potent inducer of EMT, as well as a factor for the maintenance of EMT in a variety of epithelial cells in culture; and it also contributes to tumor invasiveness in vivo6,7. Studies have indicated that tumor cells produce increased levels of active TGF-β and TGF-β receptor, resulting in an enhanced autocrine TGF-β signaling, which promotes or is required for the induction of EMT in carcinoma cells thus contributing to tumor progression8. Moreover, the TGF-β-mediated Smad signaling has been demonstrated to play an essential role in EMT associated with tumor progression7,9. In parallel, the TGF-β receptors have been shown to be able to activate non-Smad signaling pathways, such as the MAPK pathways, PI3K pathways, and Rho-like GTPase signaling pathways during TGF-β-induced EMT10,11,12. TGF-β rapidly activates the RhoA-dependent signaling pathways to induce stress fiber formation and mesenchymal characteristics during EMT in epithelial cells10,13,14.
Cyclin-dependent kinase 5 (CDK5) is a proline-directed serine/threonine kinase, playing important roles in neuronal development and differentiation, especially in synaptic plasticity and axon guidance15,16. In contrast to other known CDKs, CDK5 does not participate in the control of a typical cell cycle progression, although some studies revealed that CDK5 suppressed the neuronal cell cycle17,18,19. Functionally, CDK5 combines with p35 or p39 to form an active complex, which phosphorylates several proteins, including the focal adhesion kinase (FAK)20, talin21, peroxisome proliferator-activated receptor γ (PPARγ)22 and c-Myc23. Recent studies have discovered that CDK5 is overexpressed and plays an important role in many malignancies, including prostate cancer, pancreatic cancer, lung cancer and glioblastoma24,25,26,27,28,29,30. CDK5 catalyzes the phosphorylation of AR (androgen receptor) at Ser-81 to stabilize and to accumulate AR proteins, and subsequently to be activated to regulate the growth of prostate cancer cells25. In pancreatic cancer, CDK5 is amplified and overexpressed, and activated by mutant K-Ras; inhibition of CDK5 blocks cancer formation and progression through the suppression of Ras-Ral signaling26,27. Despite the significant progresses in studies of the roles of CDK5 in human tumorigenesis, the involvement and impact of CDK5 in the migration and invasion behavior of breast cancer cells remains uninvestigated to date.
In this study, we established that CDK5 and p35 were highly expressed in a variety of breast cancer cell lines and breast cancer tissues as compared with the para-cancer tissues. We found that CDK5 was abnormally overexpressed in clinical human breast cancer samples and was significantly correlated with several poor prognostic parameters of breast cancer. We also showed that TGF-β1 regulated CDK5 and p35 expression in human mammary epithelial cell line MCF10A. Importantly, we demonstrated that knockdown of CDK5 inhibited the TGF-β1-induced EMT, and overexpression of CDK5 resulted in a potential synergy in TGF-β1-induced EMT in MCF10A cells. Meanwhile, the shRNA-mediated silencing of CDK5 or the Roscovitine (Rv)-mediated CDK5 activity inhibition in breast cancer cell lines MDA-MB-231 and BT549 suppressed migration and invasion in vitro; and silencing of CDK5 also reduced tumor formation in nude mice in vivo. Mechanistically, we demonstrated that CDK5 participated in modulation of cancer cell migration and tumor formation through phosphorylation of FAK at Ser-732. In MDA-MB-231 and BT549 cells, overexpression of CDK5 upregulated the Ser-732 phosphorylation of FAK and evidently promoted the formation of F-actin bundles; in contrast, knockdown of CDK5 or inhibition of CDK5 kinase activity downregulated the Ser-732 phosphorylation of FAK and suppressed the formation of F-actin bundles. This study unraveled a novel function of the protein kinase CDK5 as a mediator of EMT and migration in cancer cells, as well as in tumor formation, implicating it as a potential target for prevention of tumorigenesis and metastasis.
Results
CDK5 and p35 were overexpressed in breast cancer cells and cancerous breast tissues
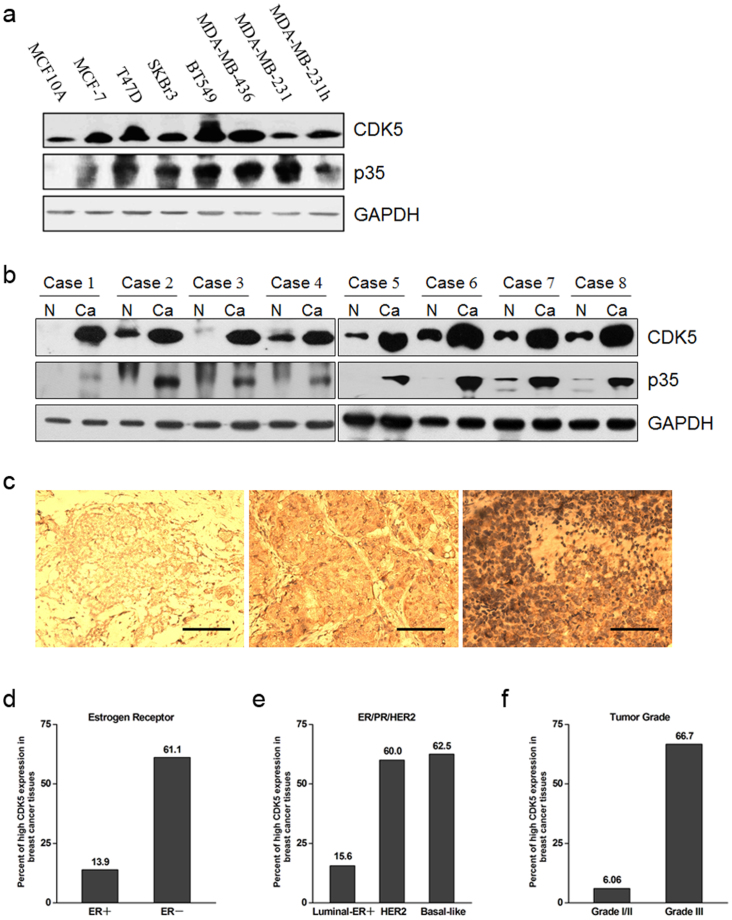
We first examined the correlation of expression of CDK5 and its co-activator p35 with breast cancer. By using immunoblotting, we showed that the protein expression levels of CDK5 and p35 were commonly higher in breast cancer cells than in non-cancerous breast epithelial MCF10A cells (Figure 1a). Meanwhile, CDK5 and p35 protein levels were also remarkably higher in breast cancer tissues than in non-cancerous surrounding tissues in all the patients examined (Figure 1b). The immunohistochemistry study of the human breast cancer specimens revealed the similar pattern characterized by a prominent increase in CDK5 protein expression in cancer tissues (Figure 1c). More specifically, analysis of the CDK5 expression in different subtypes of breast cancer specimens demonstrated the high CDK5 staining in 61.1% of the estrogen receptor negative (ER−), 60.0% of the HER2 positive (HER2+), 62.5% of the basal-like and 66.7% of the III grade breast cancer tissues (Table 1; Figure 1d–f). In contrast, only 13.9% of the ER+, 15.6% of the luminal-ER+ and 6.06% of the I/II grade breast cancer specimens exhibited high expression of CDK5 (Figure 1d–f). These data indicated that CDK5 overexpression was significantly correlated with several poor prognostic parameters of breast cancer, e.g., the ER− (P < 0.001), basal-like (P < 0.001), and high grade of malignancy (P < 0.001).
Figure 1. Enhanced expression of CDK5 and p35 in breast cancer cells and cancerous tissues.
(a) immunoblots of CDK5 and p35 protein expression in MCF10A mammary epithelial cells and breast cancer cells. (b) immunoblotting analysis of CDK5 and p35 protein expression in breast cancer tissue specimens, including non-cancerous surrounding tissues (N) and cancerous tissues (Ca). (c) immunohistochemistry of CDK5 protein in breast cancer tissue specimens. Left, weak staining; middle, moderate staining; right, strong staining. Scale bar = 100 μm. (d), (e) and (f) percentages of human breast cancer specimens with high level of CDK5 expression in different tumor subtypes and different tumor grades. Corresponding p-values analyzed by χ2 test are indicated.
Table 1. Correlation of CDK5 expression with breast tumor subtypes.
| Tumor type | Total No. of cases | No. with High-level Expression for CDK5 | Percentage with high CDK5 expression | P value |
|---|---|---|---|---|
| ER+ | 72 | 10 | 13.9 | |
| ER- | 36 | 22 | 61.1 | 8.42E-07 |
| Luminal-ER+ | 72 | 10 | 15.6 | |
| HER2 | 20 | 12 | 60.0 | 0.000081 |
| Basal-like | 16 | 10 | 62.5 | 0.000153 |
| Grade I/II | 66 | 4 | 6.06 | |
| Grade III | 42 | 28 | 66.7 | 1.47E-11 |
CDK5 and p35 overexpression occurred during TGF-β1-induced EMT, accompanied by an increase of CDK5 kinase activity
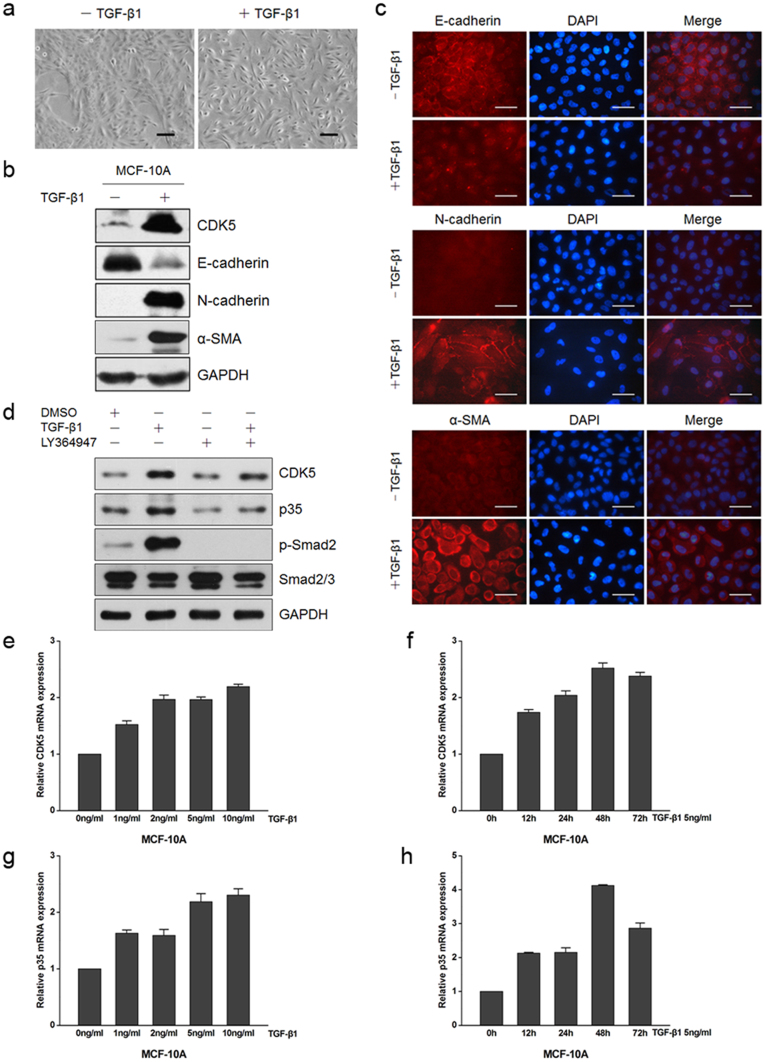
TGF-β1 has been implicated both as a potent inducer and a maintenance factor of EMT6,31. To investigate the roles of CDK5, we used TGF-β1 (5 ng/ml, 48 h) to induce EMT in immortalized non-transformed human epithelial cell line MCF10A. We observed that MCF10A cells cultured without TGF-β1 retained their cobblestone-like morphology with tight cell-cell contact, whereas cells cultured with TGF-β1 displayed an elongated fibroblast-like morphology with scattered distribution in culture (Figure 2a). We then examined both the epithelial and mesenchymal markers by using immunoblotting (Figure 2b) and immunofluorescence (Figure 2c). As can be seen, the MCF10A cells cultured with TGF-β1 exhibited a significant downregulation of epithelial marker E-cadherin; meanwhile the mesenchymal markers N-cadherin and α-smooth muscle actin (α-SMA) were dramatically upregulated. In this TGF-β1-induced EMT model, we detected the upregulation of CDK5 and p35 protein levels (Figure 2b and d); and in the meantime, we observed a simultaneous rise of the kinase activity of CDK5, as revealed by the increase of phosphorylation level of FAK at Ser-732 (Supplementary Figure S1e). Similar results were observed in HMLE (Supplementary Figure S1a and c) and MDCK (Supplementary Figure S1b and d) cells, the two prototypic cell models for TGF-β1-induced EMT study.
Figure 2. CDK5 and p35 upregulation and increased CDK5 kinase activity during TGF-β1-induced EMT in MCF10A cells.
(a) morphologic change of MCF10A cells cultured without or with TGF-β1 (5 ng/ml, 48 h), Scale bar = 100 μm. (b) immunoblotting analysis of expression of CDK5 and the epithelial marker E-cadherin, and the mesenchymal markers N-cadherin and α-SMA. (c) immunofluorescence staining for the epithelial marker E-cadherin, and the mesenchymal markers N-cadherin and α-SMA. Scale bar = 50 μm. (d) immunoblotting analysis of CDK5 and p35 protein expression in MCF10A cells without or with TGF-β1 induction and its inhibitor LY364947. The smad2/3 and p-smad2 were used as the positive control of cultured with TGF-β1. (e), (f), (g) and (h) real-time PCR analysis of CDK5 and p35 mRNA expression upon TGF-β1 treatment. Error bars represent the mean ± SD of triplicate experiments.
To further investigate the relevance of CDK5 with TGF-β1, we demonstrated that CDK5 was upregulated in response to TGF-β1 in concentration- and time-dependent manners, as determined by real-time PCR analysis (Figure 2e and f). Meanwhile, we also detected an increase in p35 mRNA level after TGF-β1 treatment (Figure 2g and h). We then used LY364947, a known TGF-β1 inhibitor, to treat MCF10A cells together with TGF-β1. We found that the effect of TGF-β1 to upregulate CDK5 and p35 proteins expression was counteracted (Figure 2d), and a simultaneous decrease in the kinase activity of CDK5 occurred (Supplementary Figure S1e).
Together, these results demonstrated that CDK5 and p35 proteins were upregulated during the TGF-β1-induced EMT in MCF10A cells, which was accompanied by an upregulation of the CDK5 kinase activity.
Knockdown of CDK5 inhibited TGF-β1-induced EMT
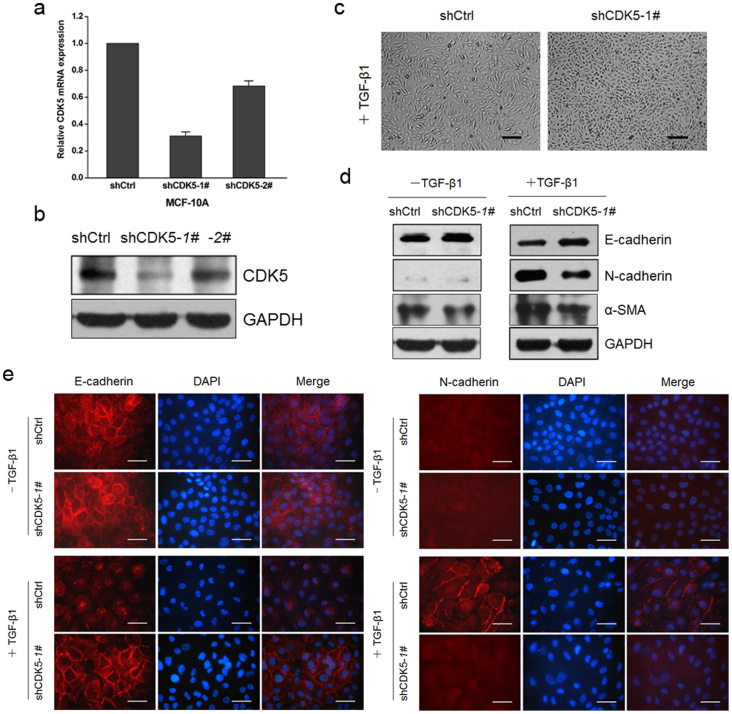
Data presented above demonstrated that CDK5 and its kinase activity were upregulated during the process of TGF-β1-induced EMT in MCF10A cells, implicating a possible role of CDK5 in EMT induction. To further validate the functional role of CDK5, we knocked down CDK5 in MCF10A cells by using the specific shRNA lentiviral infection, and the interference efficiencies of shRNAs were confirmed by real-time PCR (Figure 3a) and immunoblotting (Figure 3b). Then we added TGF-β1 into the culture medium of MCF10A cells that were stably integrated with control shRNA or shCDK5-1#. We discovered that MCF10A-shCtrl cells treated with TGF-β1 displayed an elongated fibroblast-like morphology with scattered distribution, whereas MCF10A-shCDK5-1# cells treated with TGF-β1 retained a more cobblestone-shaped epithelial morphology (Figure 3c). We then examined both epithelial and mesenchymal markers by using immunoblotting and immunofluorescence, and the results showed that treatment of MCF10A-shCDK5-1# cells with TGF-β1 resulted in increased expressions of epithelial markers E-cadherin and Occludin, and decreased expressions of mesenchymal markers N-cadherin, α-SMA, Fibronectin and Vimentin, in comparison to that in MCF10A-shCtrl cells (Figure 3d and e; Supplementary Figure S2). Thus, our loss-of-function study also pointed to a critical role of CDK5 in TGF-β1-induced EMT in MCF10A cells.
Figure 3. Knockdown of CDK5 inhibited TGF-β1-induced EMT.
(a) and (b) assessment of the repression efficiency of CDK5 mRNA (a) and protein (b) expression after retroviral infection in MCF10A cells. Error bars represent the mean ± SD of triplicate experiments. (c) morphologic change of MCF10A cells in culture with TGF-β1 after infection of shCDK5-1# or empty vector, Scale bar = 200 μm. (d) immunoblotting analysis of expression of the epithelial marker E-cadherin and the mesenchymal markers N-cadherin and α-SMA in MCF10A cultured without or with TGF-β1 after infection of shCDK5-1# or empty vector. (e) immunofluorescence staining for the epithelial marker E-cadherin and mesenchymal marker N-cadherin. Scale bar = 50 μm.
Overexpression of CDK5 resulted in a potential synergy in TGF-β1-induced EMT in MCF10A cells
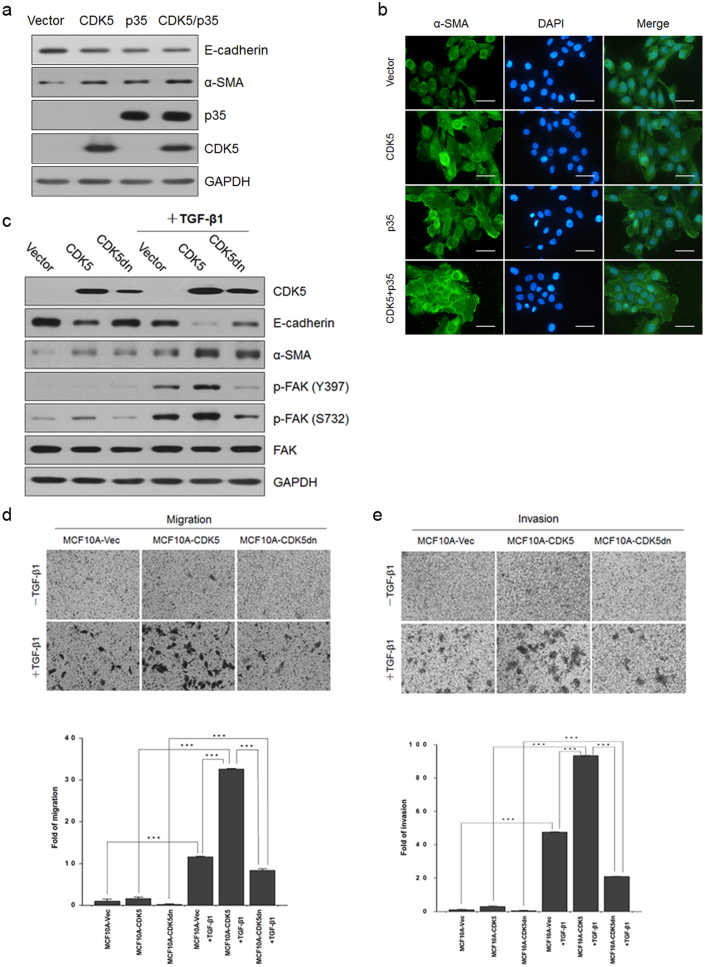
To further address the possible mechanisms of CDK5 action in TGF-β1-induced EMT, we overexpressed CDK5 and its domain negative construct (D144N; CDK5dn) that is catalytically inactive in MCF10A cells. We found that the epithelial marker E-cadherin was slightly downregulated while the mesenchymal marker α-SMA was upregulated (Figure 4a). Nevertheless, the change in cellular morphology was not detected in the meantime. Furthermore, we examined the cellular re-localization of the cytoskeleton-related mesenchymal marker α-SMA by immunofluorescence. Specifically, the α-SMA became distributed throughout the entire cells and more intercellular filaments were seen upon CDK5 or/and p35 ectopic overexpression, in contrast to the control cells (Figure 4b). Moreover, we found that the expression of EMT markers E-cadherin and α-SMA were changed markedly when TGF-β1 was added along with the overexpression of CDK5 (Figure 4c). We also determined that CDK5 had a synergistic effect with TGF-β1 to promote cell motility in MCF10A cells as revealed by cell migration and invasion assays (Figure 4d and e). Similarly, we demonstrated that inhibition of CDK5 kinase activity by CDK5dn was able to partially reverse the TGF-β1-induced EMT.
Figure 4. CDK5 had a potential synergistic effect on TGF-β1-induced EMT via CDK5 kinase activity on the phosphorylation of FAK at Ser-732.
(a) immunoblotting analysis of expression of CDK5 and p35, the epithelial marker E-cadherin, and the mesenchymal markers N-cadherin and α-SMA in MCF10A-Vector, MCF10A-CDK5, MCF10A-p35 and MCF10A-CDK5-p35 cells. (b) immunofluorescence staining for the mesenchymal marker α-SMA. Scale bar = 50 μm. (c) immunoblotting analysis of expression of CDK5, the epithelial marker E-cadherin, the mesenchymal markers α-SMA, FAK, p-FAK Y397 and p-FAK S732 in MCF10A-Vector, MCF10A-CDK5 and MCF10A-CDK5dn cells with or without TGF-β1 treatment. (d) and (e) migration (24 h; d) and invasion (60 h; e) assays in MCF10A-Vector, MCF10A-CDK5 and MCF10A-CDK5dn cells with or without TGF-β1 treatment. The mean was derived from cell counts of 5 fields, and each experiment was repeated 3 times (***, P < 0.001, compared with the control). Representative images of migrated and invaded cells are shown (upper).
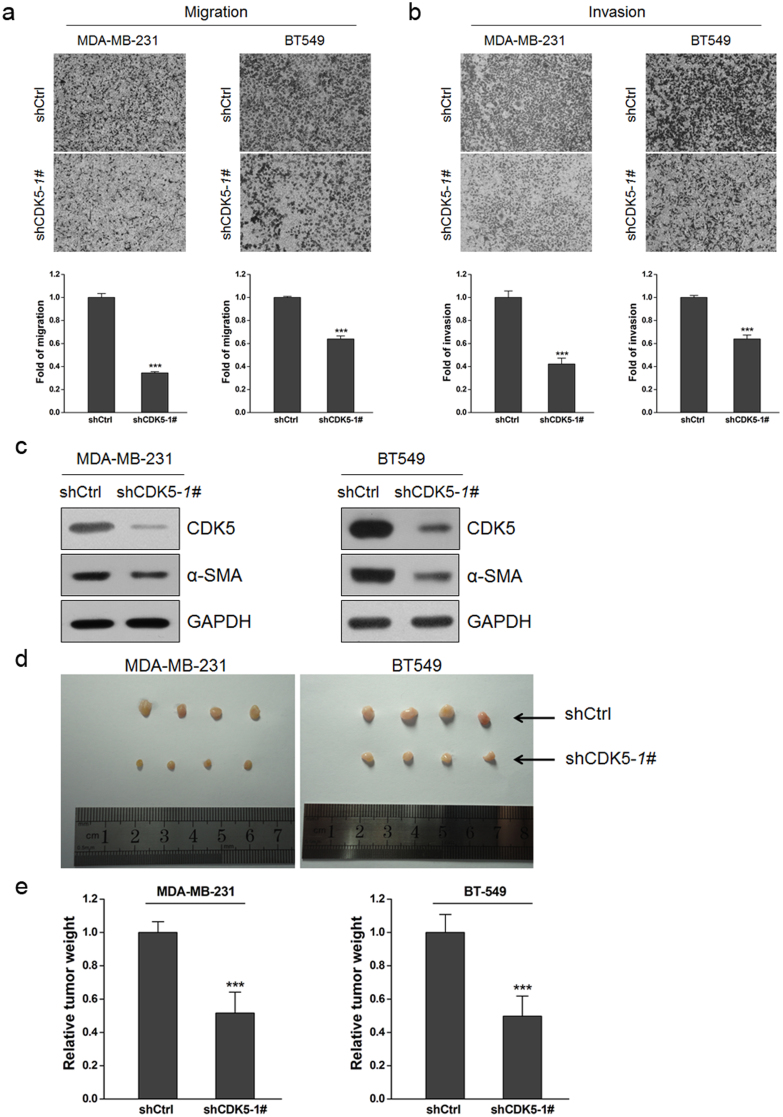
Knockdown of CDK5 expression or inhibition of CDK5 kinase activity impaired breast cancer cell motility in vitro and suppressed tumorigenesis in vivo
To investigate the functions of CDK5 in breast cancer, we knocked down CDK5 expression in two mesenchymal phenotype breast cancer cell lines MDA-MB-231 and BT549, and the silencing efficiency was confirmed by western blotting (Figure 5c). We detected no distinct changes in cell proliferation ratio after knockdown of CDK5 (Supplementary Figure S3d and e). We then examined the relevance between CDK5 and cancer cell motility, and found that the shRNA-mediated CDK5 silencing was able to significantly inhibit the migration (Figure 5a) and invasion (Figure 5b) in both MDA-MB-231 and BT549 breast cancer cells. We next tested the influence of CDK5 silencing on the EMT-related molecular markers. While we detected no apparent changes in the expression of the typical epithelial marker E-cadherin and the mesenchymal marker N-cadherin (data not shown), another important mesenchymal marker α-SMA was found remarkably downregulated upon CDK5 knockdown (Figure 5c). In order to further verify our results, the above experiments were repeated by using CDK5 kinase activity inhibitor Roscovitine (Rv), and the results were consistent with that from the knockdown studies. Specifically, addition of Rv to MDA-MB-231 and BT549 cells significantly inhibited the migration and invasion ability (Supplementary Figure S3a and b); and the mesenchymal marker α-SMA was remarkably decreased in the meantime (Supplementary Figure S3c). Moreover, the ratio of cell proliferation was declined after addition of Rv for 48 h (Supplementary Figure S3f and g). These results prompted us to speculate that CDK5 may be able to affect the configuration of the cytoskeleton in tumor cells, thereby to affect the cell morphology and migration property, as will be proven in the following experiments.
Figure 5. Knockdown of CDK5 inhibited breast cancer cell motility and tumorigenesis.
(a) and (b) migration (24 h; a) and invasion (48 h; b) assays in MDA-MB-231 and BT549 cells after infection of shCDK5-1# or empty vector. The mean was derived from cell counts of 5 fields, and each experiment was repeated 3 times (***, P < 0.001, compared with the control). Representative images of migrated and invaded cells are shown. (c) immunoblotting analysis of expression of CDK5 protein, and the mesenchymal marker α-SMA in MDA-MB-231 and BT549 cells after infection of shCDK5-1# or empty vector. (d) tumors from the BALB/c female nude mice that were subcutaneously injected with MDA-MB-231 and BT549 cells stably expressing shCDK5-1#. (e) weight of tumors at day 60 after injection. (***, P < 0.001, compared with the control). Representative images of migrated and invaded cells are shown.
Next, we used a nude mouse xenograft tumor transplantation model to investigate the role of CDK5 in tumorigenesis in vivo. The results demonstrated that the ability of tumorigenesis caused by the injection of breast cancer cells harboring the shCDK5 was significantly lower than that of the control cells, as manifested by the apparent smaller tumor size (Figure 5d) and a 50% reduction in tumor weight (Figure 5e).
Together, these data clearly indicate that knockdown of CDK5 can significantly inhibit breast cancer cell migration and invasion in vitro, and reduce the tumorigenesis ability of breast cancer cells in vivo.
The CDK5 kinase activity was essential for its function in promoting breast cancer cell motility via phosphorylation of FAK at Ser-732
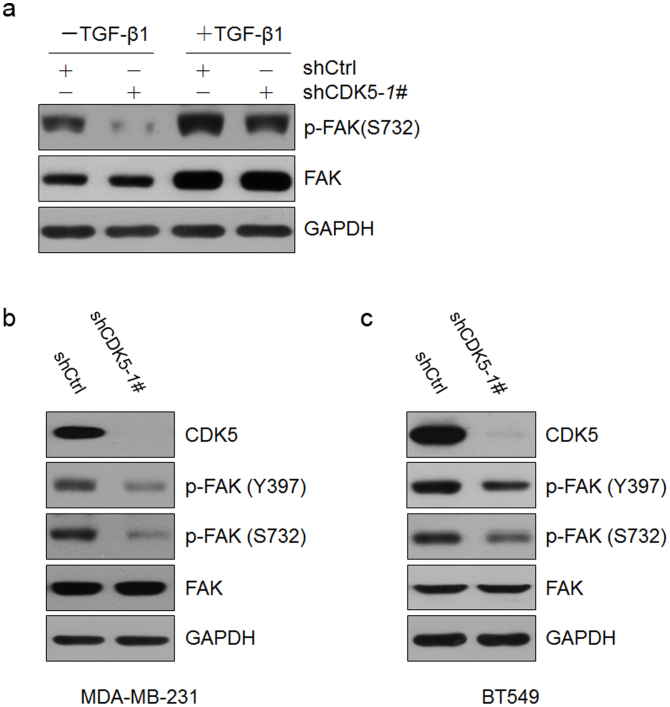
As a proline-directed serine/threonine kinase, CDK5 can phosphorylate a wide range of protein substrates, including the Focal Adhesion Kinase (FAK)19. FAK, also known as protein tyrosine kinase 2 (PTK2), is involved in cellular adhesion and spreading processes. FAK is recruited as a participant in focal adhesion dynamics between cells, and plays a role in cell motility32,33. It has been shown that overexpression of FAK leads to the inhibition of apoptosis and an increase in the prevalence of metastatic tumors; and when FAK is blocked, breast cancer cells become less metastatic due to decreased mobility34,35,36. Previous studies have established that the phosphorylation of serine 732 of FAK by CDK5 is essential for its function in cell motility37. Moreover, phosphorylation of FAK at Ser-732 is also important for microtubule organization, nuclear movement and cell migration20. Our immunoblotting assays demonstrated that both the levels of FAK and the phosphorylated FAK at Ser-732 were increased in MCF10A cells after TGF-β1 treatment (Figure 6a, bands 1 and 3). Meanwhile, knockdown of CDK5 counteracted the TGF-β1-induced an increase of FAK Ser-732 phosphorylation in MCF10A cells (Figure 6a, bands 2 and 4). We also observed that knockdown of CDK5 was unable to completely counteract the increase in the Ser-732 phosphorylation of FAK induced by TGF-β1 (Figure 6a, bands 3 and 4). Le Boeuf et al37. and Lock et al38. reported that the Rho-dependent kinase 1 could also catalyze the phosphorylation of FAK at Ser-732, and the ROCK kinase was expressed in an active state in MCF10A39, MDA-MB-23140 and BT54941 cells. Thus, ROCK may also play a role in the phosphorylation of FAK Ser-732 after CDK5 knockdown.
Figure 6. The kinase activity of CDK5 was essential for its function via phosphorylation of FAK at Ser-732.
(a) immunoblotting analysis of expression of FAK and p-FAK S732 in MCF10A cells cultured without or with TGF-β1 after infection of shCDK5-1# or empty vector. (b) and (c) immunoblotting analysis of expression of CDK5, FAK, p-FAK Y397 and p-FAK S732 in MDA-MB-231 (b) and BT549 (c) cells after infection of shCDK5-1# or empty vector.
In breast cancer cell lines MDA-MB-231 and BT549, knockdown of CDK5 also resulted in the decrease of phosphorylation level of FAK Ser-732 (Figure 6b and c). Interestingly, we observed a concurrent decrease of the phosphorylation level of FAK Tyr-397 (Figure 6b and c). A simultaneous decrease in the phosphorylation level of FAK at Ser-732 and Tyr-397 following the inhibition of CDK5 kinase activity by Rv in breast cancer cells was also observed (Supplementary Figure S4).
Since the phosphorylation of FAK at Tyr-397 has been known to dictate its function in response to integrin-mediated cell adhesion and migration and exhibit an anti-apoptosis effect34,35, we came to a deduction that the phosphorylation modification of the two FAK amino acid residues, i.e., Ser-732 and Tyr-397, may involve an interplay that represents a novel mechanism in modulating the progression of breast cancer.
CDK5 affected cytoskeletal protein F-actin remodeling depending on its kinase activity
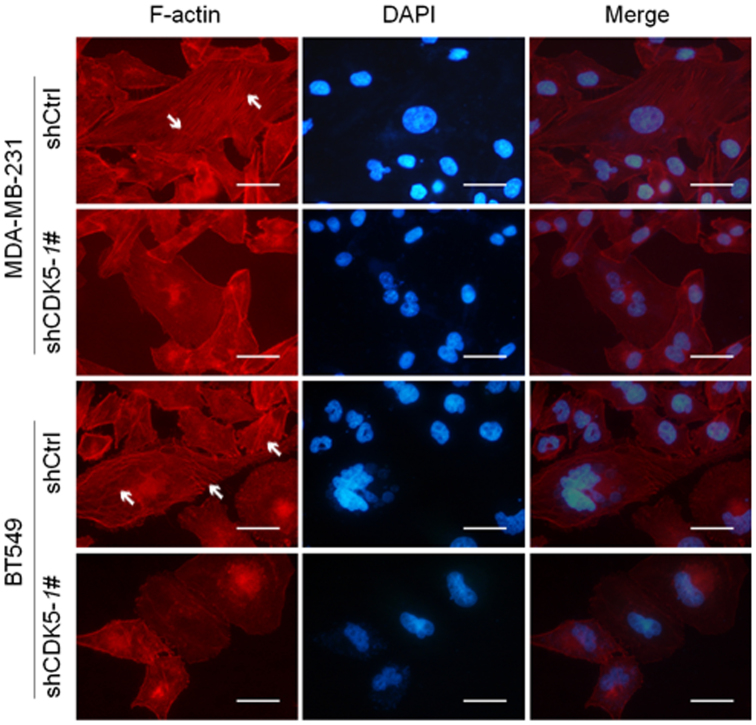
The above results from the morphological observation and molecular marker (α-SMA) study implicated a potent function of CDK5 in EMT and cell migration, possibly via modulating the cytoskeletal configuration. To provide evidence to support this assumption, we stained F-actin, an essential component of cytoskeleton, by TRITC-phalloidin in breast cancer cells MDA-MB-231 and BT549 after knockdown and overexpression of CDK5 or CDK5dn. The immunofluorescence evidenced that CDK5 knockdown caused the depolymerization and the redistribution of the cellular F-actin (Figure 7), indicating that the formation of F-actin bundles was suppressed. Meanwhile, we overexpressed CDK5 and CDK5dn in MDA-MB-231 and BT549 cells, and found further evidence to support our claims. Furthermore, we showed that overexpression of CDK5 evidently promoted the formation of F-actin bundles; in contrast, CDK5dn suppressed the formation of F-actin bundles (Supplementary Figure S4c and d), providing further evidence for the critical roles of CDK5 in the organization of actin in cytoskeleton. The experiments using the CDK5 dominant negative mutant demonstrated that CDK5 affected cytoskeletal protein F-actin remodeling depending on its kinase activity at the phosphorylation of FAK Ser-732 (Supplementary Figure S4b).
Figure 7. Knockdown of CDK5 impaired the cytoskeleton remodeling.
Immunofluorescence staining for F-actin by phalloidine in MDA-MB-231 and BT549 cells after infection of shCDK5-1# or empty vector. Scale bar = 50 μm. Arrows indicate the F-actin bundles.
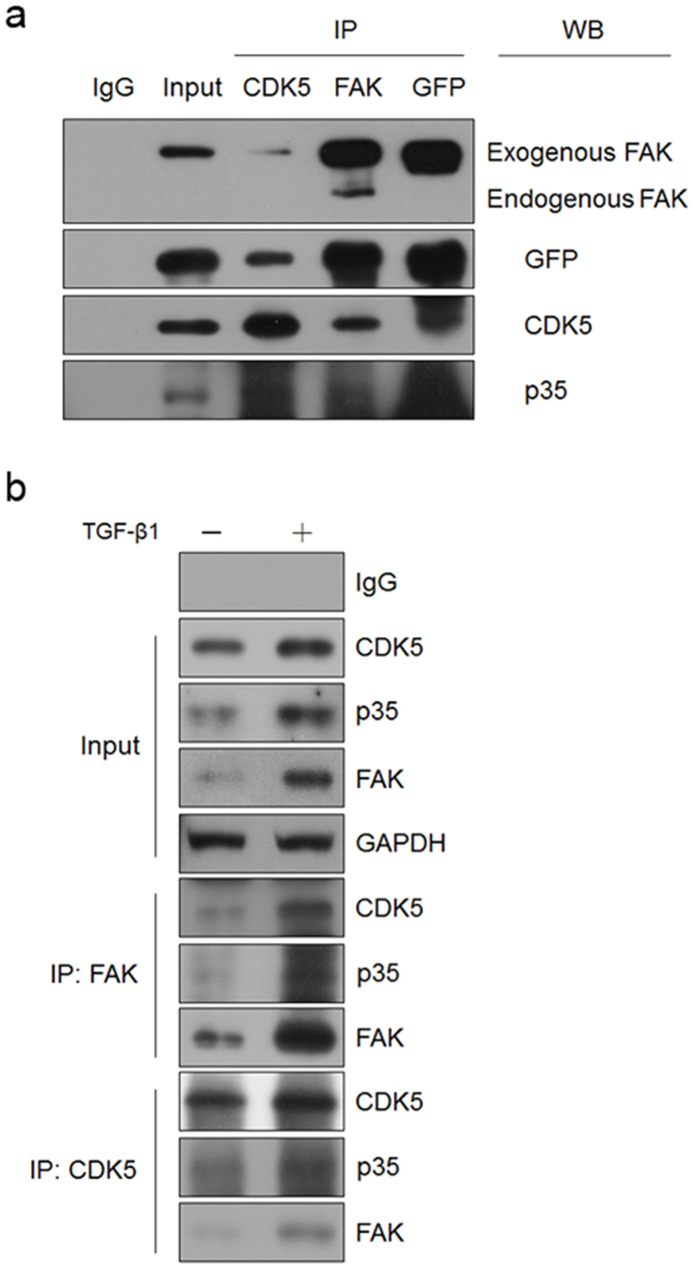
CDK5 associated with FAK in a complex in vivo
We have proven that CDK5 affects cells motility via phosphorylating FAK at Ser-732 in breast cancer cells. We next wanted to determine whether there is an interaction between CDK5 and FAK. First, we exogenously expressed CDK5 and FAK-GFP proteins in human embryonic kidney 293T cells. The whole cell lysates were then incubated with the respective specific antibodies followed by western blotting analysis. The results showed that CDK5 associated with FAK in the same complex, together with p35 protein (Figure 8a). We then studied the endogenous interaction of CDK5 and FAK by using co-immunoprecipitation (Co-IP) assay in MCF10A versus TGF-β1-induced MCF10A cells. Co-immunoprecipitation of whole cell lysates with an antibody against FAK or CDK5, followed by western blot analysis and identification of FAK, CDK5 and p35, was performed. As can be seen from Figure 8b, TGF-β1 was able to induce the expression of FAK, CDK5 and p35 proteins in MCF10A cells, and to promote the formation of the complex harboring the three proteins. Clearly, CDK5 and p35 can associate with FAK to form a complex in vivo.
Figure 8. CDK5 associated with FAK in a complex in vivo.
(a) Co-IP of CDK5, FAK and p35 in 293T cells transfected with CDK5 and FAK-GFP. (b) Co-IP of endogenous CDK5, FAK and p35 in MCF10A cells cultured without or with TGF-β1.
Discussion
In this study, we tested the hypothesis that the protein kinase CDK5 is essential for EMT and breast cancer progression. This hypothesis was postulated based on several reports that implicated the link between CDK5 and a variety of human cancer types24,25,26,27,28,29,30. However, prior to this study, the relevance between CDK5 and breast cancer development has not been investigated. The purpose of this study was to clarify the functional role of CDK5 in breast tumorigenesis and progression, with special emphasis on its role in breast cancer migration and invasion. We unraveled in this study a novel role of CDK5 in TGF-β1-induced EMT and in breast cancer progression, via modulating the phosphorylation of FAK protein at Ser-732. As a focal adhesion-associated protein kinase, FAK plays a crucial role in cancer, and its phosphorylation modification has become an attraction of investigation. Evidence from this study indicates that changes in the CDK5-dependent FAK phosphorylation are crucial for breast cancer progression.
Previous studies indicate that CDK5 regulates several processes in nervous system, including neuronal migration, actin and microtubule remodeling, axonal guidance and synaptic plasticity during nervous system development15,16,20. Recently, CDK5 has been proposed to possess other functions than that in nervous system, especially in cancer progression; these include vascular angiogenesis, cell adhesion and cell migration in several types of human cancer24,42. For example, the CDK5 gene was amplified and implicated in enhancing the malignant progression and in promoting metastasis in pancreatic cancer; this function was achieved by the action of CDK5 and its activator in concert with the mutant K-Ras and Ras-Ral signaling26,27. Similarly, in prostate cancer, the CDK5 activity was shown to control the cell motility and metastatic potential through remodeling the microtubule cytoskeleton and cellular polarity24. In contrast, several investigations have come up with somewhat different conclusions; their results suggest that suppression of CDK5 could enhance the migration of corneal epithelial cells and keratinocytes43,44. Based these previous data, we conceive that CDK5 may play distinct roles in different intracellular signal microenvironments.
In an attempt to test the function of CDK5 in EMT, we established cell lines stably expressing CDK5, p35 and CDK5-p35 genes (i.e., MCF10A-CDK5, MCF10A-p35 and MCF10A-CDK5-p35 cell lines), respectively. We examined the cellular re-localization of the cytoskeleton-related mesenchymal marker α-SMA by using immunofluorescence. In contrast to the control cells, the α-SMA became distributed throughout the entire cells and more intercellular filaments were seen upon CDK5 or/and p35 ectopic overexpression (Figure 4b). We further validated the relationship of CDK5 and cytoskeleton remodeling by overexpression of CDK5 and CDK5dn in breast cancer cell lines (Supplementary Figure S4c and d). We thus conclude that CDK5 kinase activity can influence the cytoskeleton remodeling.
It is well known that twist and snail are the classic EMT inducers45; both of which can induce EMT process in epithelial cells such as MCF10A (Figure 4a). We found in this study that an upregulated CDK5 protein level was accompanied with the changes of EMT markers (Figure 4b). This encouraged us to further examine the functional roles of CDK5 in twist- and snail-induced EMT, and the results were consistent with that in the TGF-β1-induced EMT. In MCF10A-Twist and MCF10A-Snail cells, knockdown of CDK5 expression reverse the process of EMT, as revealed by detecting the EMT markers by using both immunoblotting (Figure 4c) and immunofluorescence (Figure 4d). Collectively, based on the data both from previous studies and from our study, we think that CDK5 is a universal and important regulator of EMT in different context, presumably through different mechanisms.
A considerable deal of studies have pointed to the correlation between high expression and activity of FAK and the metastatic property and poor prognosis of cancer46,47. Moreover, FAK gene was found amplified and overexpressed in breast cancer cells and tissues32,42. FAK is generally activated by integrins and growth factors, and it regulates various signaling pathways related to cell spreading, adhesion, migration, proliferation, angiogenesis and cytoskeletal organization48,49. Integrins and growth factors are widely recognized as important regulators of TGF-β1-induced EMT and breast cancer progress1,50,51. Moreover, it has been identified that the Ser-732 residue of FAK protein is a target of phosphorylation by CDK5 activity in neurons, and this modification is implicated in microtubule organization and neuronal migration20. Inhibition of FAK at Ser-732 phosphorylation suppresses endothelial cell proliferation and angiogenesis in vitro and significantly reduces tumor angiogenesis and growth in vivo52. Similarly, transfection of MEF-FAK(−/−) cells with FAK S732A mutant gene inhibits the VEGF-induced cell migration in contrast to the wild-type FAK MEF cells37. In spite of these studies, however, the role of p-FAK Ser-732 in EMT and breast cancer progress remains unclear. We propose here that the mechanisms responsible for CDK5 function in EMT involve a decrease in p-FAK Ser-732 as a consequence of CDK5 downregulation. Significantly, we detected a simultaneous changes of the phosphorylation level of FAK at Tyr-397 along with that of p-FAK Ser-732 after knockdown and overexpression of CDK5 and inhibition of CDK5 kinase activity (Rv and CDK5dn) in breast cancer cells (Figure 6b and c; Supplementary Figure S4a and b). It has been proposed that the phosphorylation of FAK Tyr-397 site is important for FAK activity and the downstream signaling pathways32,33; our results of the simultaneous changes of p-FAK Tyr-397 and p-FAK Ser-732 are in line with this proposal. Nevertheless, Xie et al20 reported that the CDK5-deficiency markedly reduced FAK Ser-732 phosphorylation, but it did not impact on FAK Tyr-397 phosphorylation or the catalytic activity of FAK in mouse brain. Results from this study strongly suggested an intrinsic correlation between p-FAK Ser-732 and p-FAK Tyr-397 in relation to the function of the protein, especially in cancer cells, although the direct interplay between the phosphorylation modifications of the two sites needs further investigation. Additionally, we detected protein-protein interactions among CDK5, p35 and FAK in our study; and this further implicates that CDK5 directly phosphorylated FAK Ser-732, and this was accompanied by the change of the phosphorylation of FAK Try-397, which is essential for FAK function.
To summarize, this study uncovers a novel function of CDK5 in modulating the EMT process, as well as its close correlation with the malignancy of breast cancer. Our data have indicated that the CDK5 activity is necessary for the TGF-β1-induced EMT, as well as in the TWIST- and SNAIL-induced EMT, in breast epithelial cells. Mechanistically, suppression of CDK5 expression downregulates the expression of mesenchymal marker α-SMA, and restrains the phosphorylation of FAK at Ser-732, resulting in changes in cytoskeleton configuration. Therefore, CDK5 may be an important player in EMT during breast cancer cell invasion and metastasis.
Methods
Cell culture, reagents and antibodies
All cell lines, except the human mammary epithelial cell line HMLE, were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA) in 2012. MCF10A cells were cultured as previously described53 in DMEM/F12 (Gibco, Grand Island, NY, USA) containing 5% horse serum (HyClone, Logan, UT,USA), 20 μg/ml EGF (R&D Systems, Minneapolis, MN, USA), 0.5 μg/ml hydrocortisone (Sigma, St Louis, MO, USA), 0.1 μg/ml cholera toxin (Sigma), and 10 μg/ml insulin (Gibco). Breast cancer cell lines MDA-MB-231 and BT549 were grown in L-15 (Gibco) and RPMI-1640 (Gibco), respectively, supplemented with 10% fetal bovine serum (HyClone). 293T and MDCK cells were grown in DMEM (Gibco) supplemented with 10% fetal bovine serum. The HMLE cells were obtained from Dr. Robert A. Weinberg's laboratory, and were cultured as previously described54 in DMEM/F12 (Gibco) containing 10 μg/ml EGF (R&D Systems), 0.5 μg/ml hydrocortisone (Sigma) and 10 μg/ml insulin (Gibco). Appropriate amounts of antibiotics were added to the media and the cultures were maintained at 37°C in 5% CO2 atmosphere. MDA-MB-231 cells were grown in L-15 medium without CO2. Induction of EMT in MCF10A cells was initiated by addition of 5 ng/ml TGF-β1 (R&D Systems) to the medium.
CDK5 inhibitor Roscovitine (Cell Signaling Technology, Boston, MA, USA) and TGF-β1 pathway inhibitor LY364947 (sigma) were dissolved in DMSO before use.
Antibodies against the following proteins were used: CDK5 (1:500), p35 (1:500) (Santa Cruz Biotechnology, Santa Cruz, CA, USA), FAK (1:1000), phosphorylated FAK Tyr-397 (1:1000), Smad2/3 (1:1000), p-Smad2 (1:1000) (Cell Signaling Technology), E-cadherin (1:2000), N-cadherin (1:2000), Vimentin (1:6000), Fibronectin (1:5000) (BD Transduction Laboratories, Lexington, KY, USA), Occludin (1:2000) (Invitrogen, Carlsbad, CA,USA), α-SMA (1:4000) (Sigma), phosphorylated FAK Ser-732 (1:1000), GAPDH (1:5000) (Millipore, Billerica, MA, USA) and GFP (1:2000) (Abcam, Cambridge, UK).
Plasmids and virus infection
The CDK5 shRNA sequences were 1# sense 5′-GATCCCCTATAAGCCCTATCCGATGTTTCAAGAGAACATCGGATAGGGCTTATATTTTTC-3′, and 2# sense 5′-GATCCCCCCAAGCTGCCAGACTATAATTCAAGAGATTATAGTCTGGCAGCTTGGTTTTTC-3′, and the control shRNA sequence was sense 5′-GATCCCCGTGAGATCGTAGTGCGTGATTCAAGAGATCACGCACTACGATCTCAC TTTTTC-3′. The shRNAs were synthesized and subcloned into a lentiviral expression plasmid pDSL-hpUGIP (a shRNA lentiviral expression destination vector, ATCC) via the Gateway LR recombination reaction (Invitrogen). The human CDK5 expression plasmid was kindly provided by Dr. Peggy Zelenka (National Eye Institute, National Institutes of Health, Bethesda, MD, USA). The CDK5 construct was subcloned into a lentiviral expression vector pWPXLd (Addgene) by PCR using the following primers (forward primer 5′-AGCTTTGTTTAAACATGCAGAAATACGAGAAACTG-3′ and reverse primer 5′-GGAATTCCATATGCTAGGGCGGACAGAAGTCGGAGAA-3′). The FAK-GFP expression plasmid was kindly provided by Dr. Li-Huei Tsai (Howard Hughes Medical Institute, Cambridge, MA, USA). All of the plasmids were verified by sequencing.
The transfer vector pDSL-hpUGIP or pWPXLd, the packaging plasmid psPAX2 (Addgene, Cambridge, MA, USA), and the envelop plasmid pMD2.G (Addgene) were transfected into 293T cells, at the ratio of 4:3:1 for production of lentiviral particles. The supernatant was filtered through a 0.45 μ filter, and was concentrated by passing through an ultrafiltration tube (Millipore).
Western blotting and co-immunoprecipitation
For preparation of protein samples for western blotting, confluent cells were washed twice in ice-cold phosphate-buffered saline (PBS) and then scraped into RIPA lysis buffer (50 mM Tris-HCl pH 7.5, 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 1 mM EDTA, 0.1% SDS, 1 mM sodium vanadate, 1 mM NaF, 1 mM PMSF, 0.1 mg/ml pepstatin, 0.1 mg/ml leupeptin and 0.1 mg/ml aprotinin). Protein concentrations were determined by using BCA (bicinchoninic acid) Protein Assay.
Co-immunoprecipitation of exogenously and endogenously expressed proteins was performed in 293T and MCF10A cells after treatments. Cells were harvested in lysis buffer (20 mM Tris-HCl pH 8.0, 10 mM NaCl, 0.5% Nonidet P-40, 1 mM EDTA pH 8.0, 1 mM PMSF, 0.1 mg/ml pepstatin, 0.1 mg/ml leupeptin and 0.1 mg/ml aprotinin). Equal amounts of proteins were incubated overnight at 4°C with 4 μg of anti-CDK5, anti-FAK, anti-GFP, or IgG antibodies in the presence of Protein G or A beads (Millipore). The resultant complexes were washed, denatured and eluted according to the manufacturer's instruction.
Immunofluorescence
Cells were plated in 12-well chamber slides coated with poly-lysine. After treatment, cells were fixed for 20 min at room temperature in 4% paraformaldehyde and permeabilized with 0.1% Triton X-100. Cells were probed for primary antibodies and TRITC or FITC labeled secondary antibody (ZSGB-BIO) or stained for F-actin by TRITC-phalloidin (Sigma). Cell nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI, Sigma). All the images were taken under a fluorescent microscope (Nikon ECLIPSE TE2000-U).
Trans-well assay
MDA-MB-231 and BT549 cells were infected with shRNA or control viruses. Seventy-two hours after infection, the stably transfected cells were starved for 24 h, and 5 × 104 cells in serum-free (RPMI-1640/0.1% BSA or L-15/0.1% BSA) media were plated into the upper chamber with 8 μm pores (Corning, Life Sciences, Lowell, MA, USA), and DMEM containing 10% FBS was placed in the lower chamber. The chambers were coated with fibronectin (FN) (BD Biosciences, San Jose, CA, USA). For migration assay, cells were stained with crystal violet (Sigma) after incubation for 16 h. For invasion assay, the upper chambers were coated with Matrigel (BD Biosciences) and stained after 48 h. Randomly selected fields were photographed (Nikon ECLIPSE 80i) and stained cells were statistic analyzed.
RNA extraction and real-time PCR
Total RNA was extracted by using Trizol reagent (Takara, Dalian, China) following the manufacturer's instructions. Two micrograms of total RNA was used for cDNA synthesis with Oligo d(T)15 primer. Real-time PCR was carried out for detection of CDK5 on a Roche LightCycler®480. PCR reactions were run in triplicate for three independent experiments. The mean of housekeeping gene GAPDH was used as a control to normalize the variability in expression levels. Primer sequences for real-time PCR were: CDK5 forward 5′-GGGAAGGCACCTACGGAACTG-3′/CDK5 reverse 5′-GGCGGAACTCGGCACACC-3′; p35 forward 5′-ACGGTGCTGTCCCTGTCTC-3′/p35 reverse 5′-TGGCGTTCTTGCTGTTCTGT-3′; and GAPDH forward 5′-TGACCCCTTCATTGACCTCA-3′/GAPDH reverse 5′-GAGATGATGACCCTTTTGGCT-3′.
Immunohistochemistry
The single-labeling immunohistochemistry was performed using the avidin biotinylated-HRP complex (ABC) method. All tissues were fixed in 4% paraformaldehyde. Immunohistochemistry was with the paraffin-embedded tissues. The slides were de-paraffinized with xylin and re-hydrated in graded alcohol and distilled H2O. Antigen was retrieved by 1 mM EDTA (pH 8.0) at boiling temperature. After antigen retrieval, endogenous peroxidase activity was blocked by 3% hydrogen peroxide for 10 min, followed by 1 h blocking incubation in goat serum (Histostain™-Plus Kits; ZSGB-BIO). The slides were then incubated overnight at 4°C with primary antibody against Cdk5 (1:150; sc-173, Santa Cruz). Next, the slides were incubated with biotinylated antibody (Histostain™-Plus Kits; ZSGB-BIO) for 10 min at room temperature. Finally, slides were visualized by 3,3′-diaminobenzidine (DAB) staining. Tris Buffered Saline (TBS) was used in all washing steps. The slides were examined under a light microscope.
The use of breast cancer samples (108 cases) in the study was approved by the Institutional Review Board of China-Japan Friendship Hospital of Jilin University with patients' consents. The classification of the clinical breast cancer samples were based on its pathological information in medical records. (ER+: estrogen receptor positive and ER−: estrogen receptor negative; luminal-ER+: human epidermal growth factor receptor-2 negative and estrogen receptor positive, HER2+: human epidermal growth factor receptor-2 positive and basal-like: human epidermal growth factor receptor-2 negative and estrogen receptor negative; Grade I: well-differentiated, Grade II: moderately differentiated and Grade III: poorly differentiated).
Extraction and isolation of cellular proteins from breast tissues
Both breast cancer and para-cancer tissues from patients in surgery were cooled immediately on ice and subsequently stored in liquid nitrogen. Tissue specimens were partially thawed and slices were cut. Proteins were extracted from 50 mg breast tissue slices in tissue total protein lysis buffer (Sangon Biotech, shanghai, China). All steps were performed on ice. Protein concentrations were determined by BCA Protein Assay.
Tumor xenograft transplantation assay
All animal work procedures were approved by the Animal Care Committee of the Northeast Normal University, China. For xenograft animal experiments, 5 × 106 MDA-MB-231-shCDK5-1# or BT549-shCDK5-1# cells in 100 μl phosphate-buffered saline (PBS) were orthotopically injected into the backside of 8-week-old female BALB/c immunocompromised mice, and tumor growth was measured biweekly with a pair of calipers. Mice were sacrificed 60 days after injection, or when the tumors reached a static size, and the tumors were weighted statistically analyzed. The mice were obtained from the Beijing HFK Bioscience Co., Ltd. (Certification NO. SCXK (Jing) 2009–0004).
Statistical analysis
Data are presented as mean ± SD. The Student t test (2-tailed) was used to determine statistically the significance of differences between groups. P < 0.05 was considered statistically significant. CDK5 expression level in human breast cancer samples were analyzed by χ2 test. Statistical analysis was carried out using the SPSS 17.0 software.
Author Contributions
Q.L., J.Z., B.H. and J.L. designed the study; Q.L., L.L., D.X.L., B.H. and J.L. wrote and revised the manuscript; Q.L., L.L., J.Z., Y.L., J.F., P.H. and R.Y. performed research; Q.L., L.L., J.Z., L.W., Y.Z., D.X.L., B.H. and J.L. analyzed data.
Supplementary Material
CDK5 is essential for TGF-beta1-induced epithelial-mesenchymal transition and breast cancer progression_Supplemental Data
Acknowledgments
The authors thank Dr. Robert A. Weinberg (Whitehead Institute for Biomedical research, Cambridge, MA, USA) for providing the HMLE cell line, Dr. Peggy Zelenka (National Eye Institute, National Institutes of Health, Bethesda, MD, USA) for providing the pEGFP-hCDK5 expression plasmid, Dr. Barry D. Nelkin (Department of Oncology, Johns Hopkins University School of Medicine, Baltimore, MD, USA) for providing the pBI-GFP-CDK5dn expression plasmid, Dr. Carlotta Glackin (Department of Neurosciences, Beckman Research Institute of City of Hope, Duarte, CA, USA) for providing the human Twist expression plasmid, Dr. K-J Wu (Institute of Biochemistry and Molecular Biology, National Yang-Ming University, Taipei, Taiwan) for providing the human Snail expression plasmid and Dr. Li-Huei Tsai (Howard Hughes Medical Institute, Cambridge, MA, USA) for providing the FAK-GFP expression plasmid. We are grateful to the funding from the National Natural Science Foundation of China (Grant numbers 91019011, 31170719, 31071149, 31100998), and from the Fundamental Research Funds for Central Universities, and the Program for Introducing Talents to Universities (B07017).
References
- Kalluri R. & Weinberg R. A. The basics of epithelial-mesenchymal transition. J. Clin. Invest. 119, 1420–1428 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acloque H., Thiery J. P. & Nieto M. A. The physiology and pathology of the EMT. Meeting on the epithelial-mesenchymal transition. EMBO Rep. 9, 322–326 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriz W., Kaissling B. & Le Hir M. Epithelial-mesenchymal transition (EMT) in kidney fibrosis: fact or fantasy? J. Clin. Invest. 121, 468–474 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acloque H., Adams M. S., Fishwick K., Bronner-Fraser M. & Nieto M. A. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J. Clin. Invest. 119, 1438–1449 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisberg M. & Neilson E. G. Biomarkers for epithelial-mesenchymal transitions. J. Clin. Invest. 119, 1429–1437 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavadil J. & Bottinger E. P. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene 24, 5764–5774 (2005). [DOI] [PubMed] [Google Scholar]
- Pick E., Moustakas A., Kurisaki A., Heldin C. & ten Dijke P. TGF-β1 receptor/ALK-5 and Smad proteins mediate epithelial to mesenchymal transdifferentiation in NmuMG breast epithelial cells. J. Cell Sci. 112, 4557–4568 (1999). [DOI] [PubMed] [Google Scholar]
- Janda E. et al. Ras and TGF[beta] cooperatively regulate epithelial cell plasticity and metastasis: dissection of Ras signaling pathways. J. Cell Biol. 156, 299–313 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcourt U., Kowanetz M., Niimi H., Heldin C. H. & Moustakas A. TGF-beta and the Smad signaling pathway support transcriptomic reprogramming during epithelial-mesenchymal cell transition. Mol. Biol. Cell 16, 1987–2002 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhowmick N. A. et al. Transforming growth factor-β1 mediates epithelial to mesenchymal transdifferentiation through a RhoA-dependent mechanism. Mol. Biol. Cell 12, 27–36 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L., Hébert M. C. & Zhang Y. E. TGF-β receptor-activated p38 MAP kinase mediates Smad-independent TGF-β responses. EMBO J. 21, 3749–3759 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakin A. V., Tomlinson A. K., Bhowmick N. A., Moses H. L. & Arteaga C. L. Phosphatidylinositol 3-kinase function is required for transforming growth factor beta-mediated epithelial to mesenchymal transition and cell migration. J. Biol. Chem. 275, 36803–36810 (2000). [DOI] [PubMed] [Google Scholar]
- Tian Y. C., Fraser D., Attisano L. & Phillips A. O. TGF-β1-mediated alterations of renal proximal tubular epithelial cell phenotype. Am. J. Physiol. Renal Physiol. 285, F130–F142 (2003). [DOI] [PubMed] [Google Scholar]
- Burridge K. & Wennerberg K. Rho and Rac take center stage. Cell 116, 167–180 (2004). [DOI] [PubMed] [Google Scholar]
- Nikolic M., Dudek H., Kwon Y. T., Ramos Y. & Tsai L. H. The cdk5/p35 kinase is essential for neurite outgrowth during neuronal differentiation. Genes Dev. 10, 816–825 (1996). [DOI] [PubMed] [Google Scholar]
- Hawasli A. H. et al. Cyclin-dependent kinase 5 governs learning and synaptic plasticity via control of NMDAR degradation. Nat. Neurosci. 10, 880–886 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weishaupt J., Neusch C. & Bähr M. Cyclin-dependent kinase 5 (CDK5) and neuronal cell death. Cell Tissue Res. 312, 1–8 (2003). [DOI] [PubMed] [Google Scholar]
- Zhang J. et al. Cdk5 suppresses the neuronal cell cycle by disrupting the E2F1–DP1 complex. J.Neurosci. 30, 5219–5228 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero S. & Herrup K. Cyclin-dependent kinase 5 is essential for neuronal cell cycle arrest and differentiation. J.Neurosci. 25, 9658–9668 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z., Sanada K., Samuels B. A., Shih H. & Tsai L. H. Serine 732 phosphorylation of FAK by Cdk5 is important for microtubule organization, nuclear movement, and neuronal migration. Cell 114, 469–482 (2003). [DOI] [PubMed] [Google Scholar]
- Huang C. et al. Talin phosphorylation by Cdk5 regulates Smurf1-mediated talin head ubiquitylation and cell migration. Nat. Cell Biol. 11, 624–630 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J. H. et al. Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARγ by Cdk5. Nature 466, 451–456 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo H. R., Kim J., Bae S., Soh J. W. & Lee Y. S. Cdk5-mediated phosphorylation of c-Myc on Ser-62 is essential in transcriptional activation of cyclin B1 by cyclin G1. J. Biol. Chem. 283, 15601–15610 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strock C. J. et al. Cyclin-dependent kinase 5 activity controls cell motility and metastatic potential of prostate cancer cells. Cancer Res. 66, 7509–7515 (2006). [DOI] [PubMed] [Google Scholar]
- Hsu F. N. et al. Regulation of androgen receptor and prostate cancer growth by cyclin-dependent kinase 5. J. Biol. Chem. 286, 33141–33149 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann G. et al. Inhibiting the cyclin-dependent kinase CDK5 blocks pancreatic cancer formation and progression through the suppression of Ras-Ral signaling. Cancer Res. 70, 4460–4469 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers J. P. et al. Cyclin-dependent kinase 5 is amplified and overexpressed in pancreatic cancer and activated by mutant K-Ras. Clin. Cancer Res. 17, 6140–6150 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demelash A. et al. Achaete-scute homologue-1 (ASH1) stimulates migration of lung cancer cells through Cdk5/p35 pathway. Mol. Biol, Cell 23, 2856–2866 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. L. et al. Expression of CDK5/p35 in resected patients with non-small cell lung cancer: relation to prognosis. Med. Oncol. 28, 673–678 (2011). [DOI] [PubMed] [Google Scholar]
- Liu R. et al. Cdk5-mediated regulation of the PIKE-A-Akt pathway and glioblastoma cell invasion. Proc. Natl. Acad. Sci. U S A 105, 7570–7575 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A. N. M. et al. Integration of Ras subeffector signaling in TGF-β mediated late stage hepatocarcinogenesis. Carcinogenesis 26, 931–942 (2005). [DOI] [PubMed] [Google Scholar]
- Luo M. & Guan J. L. Focal adhesion kinase: a prominent determinant in breast cancer initiation, progression and metastasis. Cancer Lett. 289, 127–139 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra S. K. & Schlaepfer D. D. Integrin-regulated FAK–Src signaling in normal and cancer cells. Curr. Opin. Cell Biol. 18, 516–523 (2006). [DOI] [PubMed] [Google Scholar]
- Sonoda Y. et al. Anti-apoptotic role of focal adhesion kinase (FAK) Induction of inhibitor-of-apoptosis proteins and apoptosis suppression by the overexpression of FAK in a human leukemic cell line, HL-60. J. Biol. Chem. 275, 16309–16315 (2000). [DOI] [PubMed] [Google Scholar]
- Chan K. T., Cortesio C. L. & Huttenlocher A. FAK alters invadopodia and focal adhesion composition and dynamics to regulate breast cancer invasion. J. Cell Biol. 185, 357–370 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebl J. et al. Cyclin-dependent kinase 5 regulates endothelial cell migration and angiogenesis. J. Biol. Chem. 285, 35932–35943 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Boeuf F., Houle F., Sussman M. & Huot J. Phosphorylation of focal adhesion kinase (FAK) on Ser732 is induced by rho-dependent kinase and is essential for proline-rich tyrosine kinase-2–mediated phosphorylation of FAK on Tyr407 in response to vascular endothelial growth factor. Mol. Biol, Cell 17, 3508–3520 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock F. E., Ryan K. R., Poulter N. S., Parsons M. & Hotchin N. A. Differential regulation of adhesion complex turnover by ROCK1 and ROCK2. PLoS One 7, e31423 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overholtzer M. et al. A nonapoptotic cell death process, entosis, that occurs by cell-in-cell invasion. Cell 131, 966–979 (2007). [DOI] [PubMed] [Google Scholar]
- Liu S., Goldstein R. H., Scepansky E. M. & Rosenblatt M. Inhibition of rho-associated kinase signaling prevents breast cancer metastasis to human bone. Cancer Res. 69, 8742–8751 (2009). [DOI] [PubMed] [Google Scholar]
- Amiri A. et al. eEF1A2 activates Akt and stimulates Akt-dependent actin remodeling, invasion and migration. Oncogene 26, 3027–3040 (2006). [DOI] [PubMed] [Google Scholar]
- Luo M. et al. Mammary epithelial-specific ablation of the focal adhesion kinase suppresses mammary tumorigenesis by affecting mammary cancer stem/progenitor cells. Cancer Res. 69, 466–474 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C. Y., Stepp M. A., Fariss R. & Zelenka P. Cdk5 regulates activation and localization of Src during corneal epithelial wound closure. J. Cell Sci. 117, 4089–4098 (2004). [DOI] [PubMed] [Google Scholar]
- Nakano N. et al. CDK5 regulates cell–cell and cell–matrix adhesion in human keratinocytes. Br. J. Dermatol. 153, 37–45 (2005). [DOI] [PubMed] [Google Scholar]
- Mani S. A. et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133, 704–715 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAO H. et al. Focal adhesion kinase as potential target for cancer therapy (Review). Oncol. Rep. 22, 973–979 (2009). [DOI] [PubMed] [Google Scholar]
- Parsons J. T., Slack-Davis J., Tilghman R. & Roberts W. G. Focal adhesion kinase: targeting adhesion signaling pathways for therapeutic intervention. Clin. Cancer Res. 14, 627–632 (2008). [DOI] [PubMed] [Google Scholar]
- Chan P. C., Chen S. Y., Chen C. H. & Chen H. C. Crosstalk between hepatocyte growth factor and integrin signaling pathways. J. Biomed. Sci. 13, 215–223 (2006). [DOI] [PubMed] [Google Scholar]
- Sieg D. J. et al. FAK integrates growth-factor and integrin signals to promote cell migration. Nat. Cell Biol. 2, 249–256 (2000). [DOI] [PubMed] [Google Scholar]
- Margadant C. & Sonnenberg A. Integrin–TGF-β crosstalk in fibrosis, cancer and wound healing. EMBO Rep. 11, 97–105 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotzmann J. et al. A crucial function of PDGF in TGF-β-mediated cancer progression of hepatocytes. Oncogene 25, 3170–3185 (2006). [DOI] [PubMed] [Google Scholar]
- Park A. Y. J., Shen T. L., Chien S. & Guan J. L. Role of focal adhesion kinase Ser-732 phosphorylation in centrosome function during mitosis. J. Biol. Chem. 284, 9418–9425 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnath J., Muthuswamy S. K. & Brugge J. S. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods 30, 256–268 (2003). [DOI] [PubMed] [Google Scholar]
- Elenbaas B. et al. Human breast cancer cells generated by oncogenic transformation of primary mammary epithelial cells. Genes Dev. 15, 50–65 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CDK5 is essential for TGF-beta1-induced epithelial-mesenchymal transition and breast cancer progression_Supplemental Data