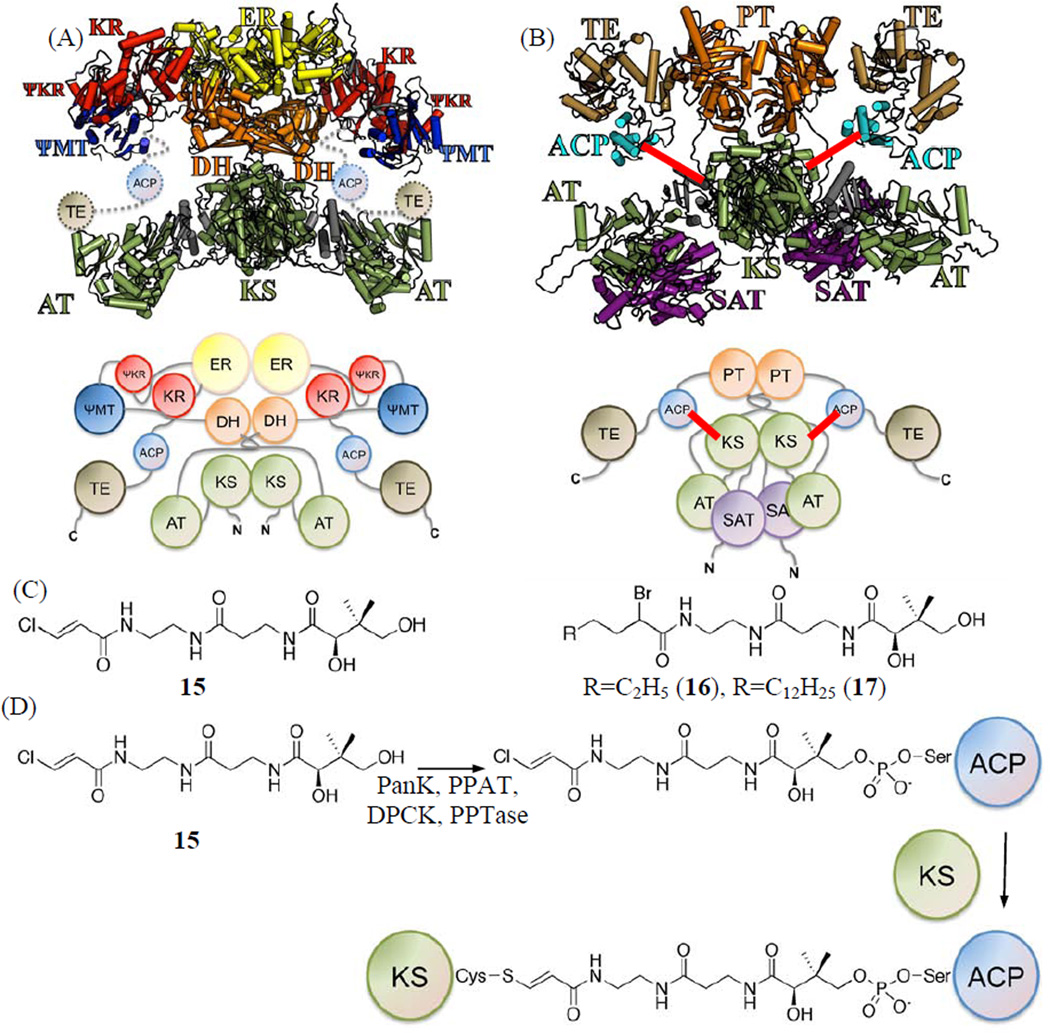
Figure 2.
(A) Crystal structure and cartoon of porcine FAS model as a homo-dimer, where KS is located at the center as a dimer. (B) Protein sizing studies of PksA supports an overall architecture similar to that of porcine FAS, where KS also exists as a dimer, and two ACPs on either side of the torso as shown in this homology model and cartoon. The cross-linking between KS and ACP is shown as red lines. (C) Pantetheinylated probes with a chloroacryl 15, 2-bromo hexyl 16 or 2-bromo palmitoyl 17 chemical groups that target the nucleophilic cysteine of the KS domain. (D) Chemo-enzymatic attachment of 15, 16 or 17 to ACP is followed by KS crosslinking the nucleophilic cysteine of the KS domain.