Figure 3.
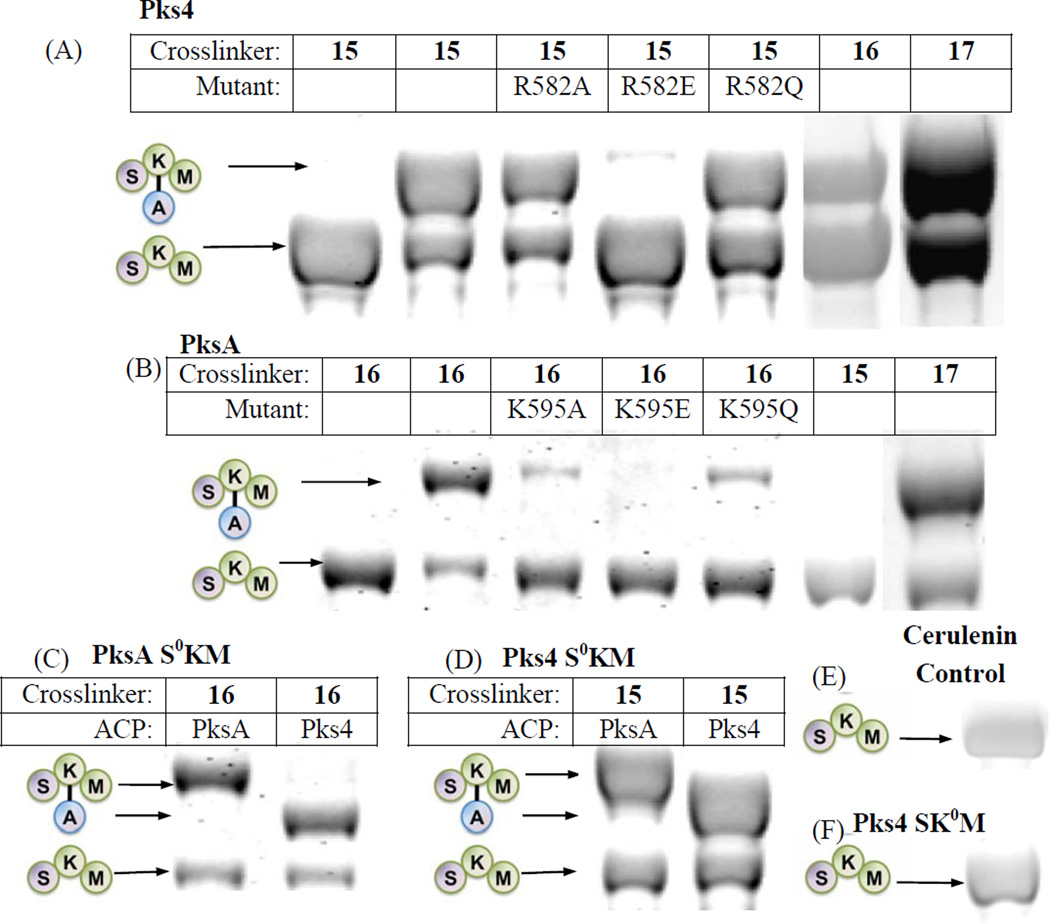
see also Figures S1–6. SKM crosslinking gels. (A) Pks4 ACP crosslinked to Pks4 S0KM and KS surface mutants. Pks4 can crosslink by crosslinkers with acyl chains ranging from 2 – 16 carbons (15 – 17). Surface mutations of Pks4 R582 (and K593 in the SI, Fig. S1A) abolished crosslinking when the side chain charge is reversed (R582E), which can be rescued by mutation to a polar amino acid (R582Q). (B) PksA ACP crosslinked to PksA S0KM and additional KS surface mutants. PksA requires a minimum of 6 carbons in the acyl chain of the crosslinkers (16–17). Reversing the charge of K595 (K595E) abolishes crosslinking, which can be rescued by mutation to a polar amino acid (K595Q). Similar results were found for K584 mutants (detailed in the SI, Fig. S1C) When comparing (A) and (B) or PksA vs Pks4, the alanine mutation do not necessarily abolish protein-protein interactions. Rather, the reversal of charge (the mutation to glutamate) effectively abolishes the KS-ACP interactions. The difference is likely due to the distinct character at the protein interface of different PKSs. (C) A comparison of PksA S0KM crosslinked to PksA and Pks4 ACP, showing comparable crosslinking efficiency for the two ACPs from NR-PKSs. (D) A comparison of Pks4 S0KM crosslinked to PksA and Pks4 ACP, showing comparable crosslinking efficiency for the two ACPs from NR-PKSs. (E) The negative control of cerulenin, which binds KS and prevents crosslinking. The result supports that the KS active site must be accessible for crosslinking to occur. (F) As an additional control, the KS0 mutant abolishes crosslinking, supporting that KS active site must be present for crosslinking to occur.