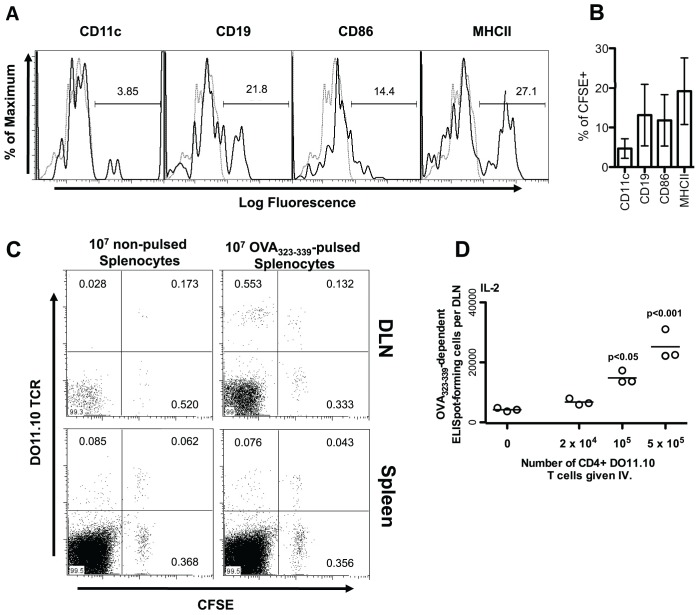
Figure 1. Cultured splenocytes migrate to the popliteal lymph node, present peptide, and activate peptide-specific CD4 T cells, following subcutaneous injection.
(A and B) BALB/c splenocytes were cultured overnight in the presence of 1 µg/mL LPS. Cells were harvested and labeled with 5 µM CFSE. After thorough washing, 107 splenocytes were re-suspended and injected subcutaneously into the footpad and lower leg of normal BALB/c mice. After 48 hours, the popliteal lymph nodes of mice were harvested and stained with fluorophore-conjugated antibodies to detect the indicated cell surface proteins. Stained cells were analyzed by flow-cytometry. The expression of individual cell surface proteins is shown for CFSE positive cells. Black histograms show the fluorescence of cells stained with the indicated antibody. Grey, dotted, histograms show the fluorescence of cells stained with a matched fluorophore-conjugated isotype-matched control antibody. (A) Representative histograms are shown. Percentages of stained cells falling within the gated region of the total number of cells within the given plot are shown. (B) Percentages of CFSE+ cells also expressing the indicated marker is shown as the mean +/− standard deviation from individual mice (n = 3). (C) 107 Splenocytes from DO11.10 mice were labeled with 5 µM CFSE, washed, and injected intravenously into BALB/c mice. Twenty-four hours later these mice were injected subcutaneously in the footpad and lower leg with BALB/c splenocytes cultured overnight in the presence or absence of 50 µM OVA323–339. Six days post-injection, draining popliteal lymph nodes (DLN) and spleens from injected mice were harvested and stained with a fluorophore-conjugated DO11.10 TCR-specific antibody. The level of CFSE staining and numbers of DO11.10 positive cells were assessed by flow cytometry. Percentages of cells falling within the gate out of the total number of cells within the given plot are shown. (D) One day prior to injection of pulsed splenocytes, BALB/c mice were injected with the indicated number of MACS purified CD4+ DO11.10 T cells. BALB/c slpenocytes were cultured overnight in the presence of 50 µM OVA323–339. Following harvest and extensive washing, 107 peptide-pulsed splenocytes in 50 µL Leibovitz media were injected into the footpad and lower leg of normal BALB/c mice on days 0, 3, and 6. Nine days after the initial injection of pulsed splenocytes, the OVA323–339-specific cytokine producing cells in the draining popliteal lymph node (DLN) were enumerated by ELISpot assay. Each data point represents the number of specific ELISpot-forming cells in the popliteal lymph node of a single mouse. P-values indicate the probability that the number of adoptively transferred DO11.10 CD4 T cells does not increase the number of cytokine producing CD4 T cells generated as assessed by one way ANOVA. Post-hoc analysis was employed to compare all groups to that in which mice were given no DO11.10 T cells. The results of a single representative experiment, of two similar experiments, are shown.