Abstract
In this research, the conditions for extraction of phenolics from leaves of Ficus virens were optimized using response surface methodology (RSM). The extraction abilities of phenolics (EAP) and flavonoids (EAF), the 2,2-diphenyl-1-pierylhydrazyl (DPPH) free-radical scavenging potential, and the ferric reducing/antioxidant power (FRAP) were used as quality indicators. The results of single-factor experiments showed that temperature, ethanol concentration, extraction time, and the number of extraction cycles were the main influencing variables, and these provided key information for the central composite design. The results of RSM fitted well to a second degree polynomial model and more than 98% of the variability was explained. The ideal extraction conditions for EAP, EAF, DPPH free-radical scavenging potential, and FRAP were obtained. Considering the four quality indicators overall, the ideal extraction conditions were 58% ethanol at 57 °C for 37 min with three extraction cycles. At the ideal extraction conditions, the values of EAP, EAF, DPPH free-radical scavenging potential, and FRAP were 5.72%, 3.09%, 58.88 mg ascorbic acid equivalent (AAE)/g dry weight (DW), and 15.86 mg AAE/g DW, respectively. In addition, linear correlations were observed between EAP, EAF, and antioxidant potential.
Keywords: Ficus virens, Phenolics, Flavonoids, Antioxidants, Response surface methodology (RSM)
1. Introduction
Ficus virens is a medium-sized fig tree. Various plant parts of Ficus species, such as leaves, stems, bark, roots, flowers, and seeds, have long been used as drugs in traditional medicine systems (Khan et al., 2011). Investigation of F. virens revealed that phenolic compounds form the major phytochemical components of the leaves and are responsible for the excellent antioxidant capacity of extracts. Furthermore, the extracts exhibit dose-dependent antioxidant activity (Abdel-Hameed, 2009; Shi et al., 2011). In recent decades, there has been increasing interest in finding naturally occurring antioxidants that can be introduced into our diet or be used as natural drugs to replace synthetic antioxidants, the use of which is being restricted due to their carcinogenicity (Sasaki et al., 2002). Antioxidative phytochemicals, especially phenolic compounds found in vegetables, fruits, and medicinal plants, have received increasing attention due to their potential role in the prevention of human diseases (Cai et al., 2004). However, methods to optimize the extraction abilities of phenolics (EAP) and flavonoids (EAF) from the leaves of F. virens have not been investigated. Thus, to make better use of such a good source of antioxidants, it is necessary to maximize the extraction of antioxidants from the leaves of F. virens.
Considering the composition of natural sources of phenolic compounds, as well as their structure and physicochemical properties, a universal extraction protocol is not conceivable and a specific extraction procedure must be designed and optimized for each phenolic source (Contini et al., 2008; Thoo et al., 2010). In the present research, ethanol and water were adopted as the extraction media because of their environmentally friendly effects and non-toxicity for human health. Among various factors contributing to the efficiency of the solvent extraction process and the recovery of antioxidant compounds from natural materials, ethanol concentration, extraction time, extraction temperature, the number of extraction cycles, and the solid-to-solvent ratio are often investigated (Yang et al., 2009; Wu et al., 2011).
The single-factor experiment is a classical optimization method. Despite being time-consuming and unable to provide information on the interactions among different factors (Zhang et al., 2007), this approach has provided fundamental information on the ranges of factors that show significant effects (P<0.05) on the ability to extract phenolic compounds from the leaves of F. virens. Response surface methodology (RSM) can overcome the difficulties encountered in single-factor experiments. It allows for the evaluation of the combined effects of all the factors and determines a wide region in which the results are valid (Pérez-Jiménez et al., 2008). Therefore, in our investigation, we first used single-factor experiments to obtain the minimum and maximum response values for each factor. This information was then applied in RSM to generate a central composite design.
The aim of this research was to investigate the effects of temperature, extraction time, ethanol concentration, solid-to-solvent ratio, and number of extraction cycles on EAP, EAF, 2,2-diphenyl-1-pierylhydrazyl (DPPH) free-radical scavenging potential, and ferric reducing/antioxidant power (FRAP) of extracts from F. virens leaves and to optimize the extraction conditions. To our knowledge, there have been few previous reports on the optimization of the extraction of phenolics and antioxidants by single-factor experiments and RSM simultaneously.
2. Materials and methods
2.1. Plant materials
Leaves of F. virens were collected from the campus of Xiamen University, China, immediately freeze-dried and then ground in a cutting mill (model BL301D5; Saikang, China) to pass through 100 mesh sieves. The resulting fine powder was stored at −20 °C in a freezer prior to analysis.
2.2. Chemicals
Water used in this experiment was purified on a Millipore Milli-Q apparatus (TGI Pure 110 Water Systems, USA). 2,4,6-Tripyridyl-S-triazine, DPPH, gallic acid (GA), catechin, and ascorbic acid were purchased from Sigma-Aldrich. Other solvents used were of analytical grade.
2.3. Selection of relevant variables and experimental ranges
The leaf powder (3.0 g) was extracted under static conditions for phenolics by water bath extraction. The experimental values of the independent variables are given in Table 1. Samples were added to a beaker after the desired temperature was reached. When a variable was not studied, it was kept constant. The constant values for ethanol concentration, extraction time, extraction temperature, and solid-to-solvent (g/ml) ratio were 70%, 50 min, 40 °C, and 3:40, respectively. The extract was filtered through a funnel with 0.45 micron filter paper by applying a reduced pressure of 20 mmHg by means of a circulating water pump (Model A-1000S, EYELA, China). Each experiment was done three times.
Table 1.
Experimental values of the independent variables (except for the number of extraction cycles) for the single-factor experiment
| No. | c e (%) | t e (min) | T e (°C) | r STS (g/ml) |
| 1 | 30 | 10 | 30 | 3:20 |
| 2 | 50 | 20 | 40 | 3:30 |
| 3 | 60 | 30 | 50 | 3:40 |
| 4 | 70 | 40 | 60 | 3:50 |
| 5 | 90 | 50 | 70 | 3:60 |
c e: ethanol concentration; t e: extraction time; T e: extraction temperature; r STS: solid-to-solvent ratio. The experiment was carried out under static conditions in a beaker covered with a film to prevent loss of solvent, so the volume of the extract is not given
2.4. Design of statistical experiments
The effects on EAP, EAF, and antioxidant potential of extraction variables including ethanol concentration, extraction temperature, extraction time, solid-to-solvent ratio, and the number of extraction cycles were investigated by a single-factor method. The major influencing factors were identified from the results, and then RSM was used to design the experimental project. The Design Expert 7.0 software package was used to establish a mathematical model and obtain the optimum conditions of extraction. The variables were coded according to the equation:
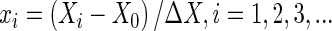 , ,
|
(1) |
where xi is the (dimensionless) coded value of the variable Xi, X 0 is the value of Xi at the center point, and ΔX is the step change. Table 2 shows the actual design of the experiment. The behavior of the system was explained by the following second degree polynomial equation:
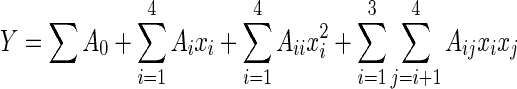 , ,
|
(2) |
where Y is the response, A 0 is the constant coefficient, and Ai, Aii, and Aij are the linear, quadratic, and interactional coefficients, respectively.
Table 2.
Experimental values and coded levels of the independent variables used for the 30-full factorial design
| xi | X 1 (%) | X 2 (min) | X 3 (°C) | X 4 |
| −1 | 40 | 30 | 40 | 1 |
| 0 | 60 | 40 | 50 | 2 |
| 1 | 80 | 50 | 60 | 3 |
xi: coded variable level; X 1: ethanol concentration; X 2: extraction time; X 3: extraction temperature; X 4: number of extraction cycles
2.5. Determination of the optimum conditions
Design Expert 7.0 was set to search for the optimum desirability of the response variables, i.e., the maximum EAP, EAF, DPPH free-radical scavenging potential, and FRAP values.
2.6. Determination of phenolic content
The total phenolic content was determined using the Folin-Ciocalteu method as described by Wei et al. (2010). Briefly, 0.2 ml of extract and 0.3 ml dH2O were mixed with 0.5 ml of Folin-Ciocalteu reagent; 2.5 ml of sodium carbonate (20%, w/w) solution was added to the mixture. After 30 min at room temperature, the absorbance was measured at 725 nm using a DU800 spectrophotometer (Beckman Coulter Inc., CA, USA) and a calibration curve was drawn using data from standard solutions of GA ranging from 0.05 to 0.40 mg/ml. The total phenolic content of the extract was calculated and expressed as milligram GA equivalents per gram of dry weight (mg GAE/g DW) based on the GA standard curve (y=6.35A−0.052, R²=0.9994, where y is the content in mg/ml and A is the absorbance). The EAP was calculated based on the value of the phenolic content.
2.7. Determination of flavonoid content
The flavonoid content of the crude extract was estimated according to the procedures described by Jia et al. (1999) with slight modifications. The reaction mixture contained the crude extract (0.2 ml), deionized water (3.35 ml), and 0.05 g/ml sodium nitrite (0.15 ml). The mixture was set still for 5 min, and then 0.3 ml of 0.1 g/ml aluminum chloride was added. After 6 min, 1 ml of sodium hydroxide (1 mol/L) solution was added and mixed. Immediately, the absorbance was measured at 510 nm against a blank and catechin solution (0.1−1.0 mg/ml), which was used as a standard (y=1.24A−0.0063, R²=0.9995, where y is the content in mg/ml and A is the absorbance). The flavonoid content was then expressed as mg catechin equivalents (CE)/g DW. The EAF was calculated based on the value of the flavonoid content.
2.8. Antioxidant potential assay
DPPH free-radical scavenging potential and the FRAP assay were used to analyze the antioxidant potential of the extract, as previously recommended by Thaipong et al. (2006).
The DPPH free-radical scavenging potential was measured according to the method of Brand-Williams et al. (1995) with some modifications. A sample (100 μl) was added to DPPH (3 ml) methanolic solution (0.1 mol/L). After 30 min, the absorbance was measured at 517 nm using a spectrophotometer. A calibration curve was drawn using data from standard solutions of ascorbic acid (0.015–0.250 mg/ml). The DPPH free-radical antioxidant potential, expressed in mg ascorbic acid equivalents (AAE)/g DW, was derived from the standard curve (y=−0.23A+0.27, R²=0.9941, where y is the content in mg/ml and A is the absorbance).
The FRAP assay was done according to the method described by Benzie and Strain (1996). Briefly, 3 ml of FRAP reagent, prepared freshly, was mixed with 100 μl of the test sample, or methanol (for the reagent blank). The FRAP reagent was prepared from acetate buffer (300 mmol/L, pH 3.6), ferric chloride (20 mmol/L) and 2,4,6-tripyridyl-S-triazine (10 mmol/L) made up in hydrochloric acid (40 mmol/L). These three solutions were mixed together in the ratio of 10:1:1 (v/v/v). The absorbance of the reaction mixture at 593 nm was measured spectrophotometrically after incubation at 25 °C for 5 min. The FRAP values, expressed in mg AAE/g DW, were derived from a standard curve (ascorbic acid 0.015–0.250 mg/ml, y=0.15A−0.0063, R²=0.9998, where y is the content in mg/ml and A is the absorbance).
2.9. Statistical analysis
All determinations were carried out at least in triplicate and values were expressed as mean±standard deviation (SD). Statistical analyses were performed using GraphPad Prism 5.0 (GraphPad Software Inc.) and Design Expert 7.0 (Stat-Ease Inc.). One-way analysis of variance (ANOVA) was performed using GraphPad Prism 5.0. Comparisons between samples were calculated using Tukey’s test for independent observations. Differences were considered significant at P<0.05. Correlations between polyphenol contents and antioxidant potential were established by linear regression analysis.
3. Results and discussion
3.1. Effects of ethanol concentration on EAP, EAF, and antioxidant potential
Cell vacuoles contain most free phenolic compounds, while in the cell wall, most lignin, flavonoids, and insoluble polyphenols are conjugated to sugars, cell wall carbohydrates, organic acids, proteins, and polysaccharides (Dixon and Paiva, 1995). A proper concentration of ethanol can access cells, while high concentrations cause protein denaturation, which prevents the dissolution of polyphenols and influences the extraction rate.
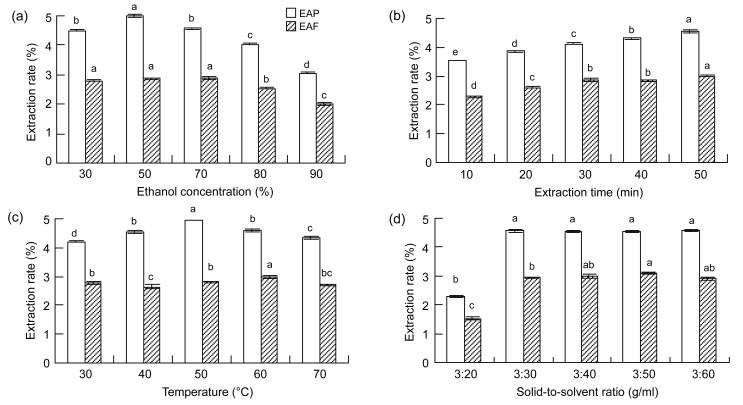
The EAP under the different concentrations of ethanol investigated ranged from (3.06±0.04)% to (4.95±0.06)%. For EAF, the extraction rates ranged from (2.88±0.05)% to (2.01±0.04)%. One-way ANOVA showed a significant effect (P<0.0001) of ethanol on EAP and EAF, which was confirmed by Tukey’s test (Fig. 1a). Changes in ethanol concentration modify the physical properties of the solvent including its density, dynamic viscosity, and dielectric constant (Cacace and Mazza, 2003b). Therefore, the polarity of a solvent is determined by its concentration and a certain concentration achieves a high extraction rate (Spigno et al., 2007; Bucić-Kojić et al., 2009).
Fig. 1.
Influences of ethanol concentration (a), extraction time (b), extraction temperature (c), and solid-to-solvent ratio (d) on EAP and EAF from leaves of F. virens
Data are expressed as mean±SD, n=3. The same letters in the columns indicate no significant difference (Tukey’s test, P>0.05)
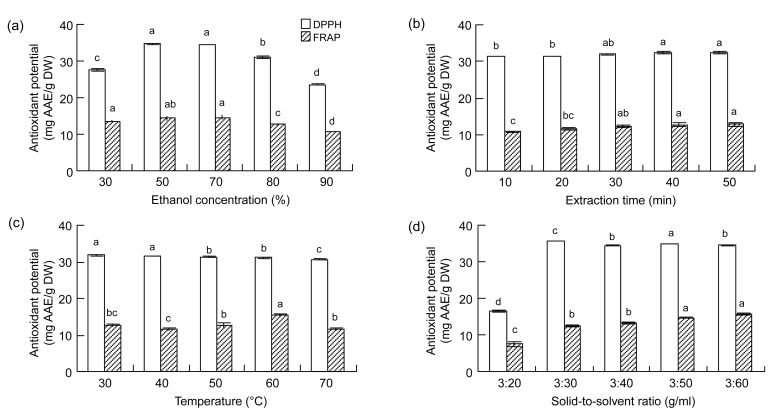
The antioxidant properties of the extract generated were assessed by two representative indices: the DPPH free-radical scavenging potential and the FRAP. Under different ethanol concentrations, the DPPH free-radical scavenging potential of the extract increased at first as ethanol concentration increased from low levels, and then decreased after reaching a maximum of (34.77±0.14) mg AAE/g DW at 50% ethanol. The trend for FRAP showed a similar tendency, with the highest value of (14.52±0.49) mg AAE/g DW at 50% ethanol. One-way ANOVA analysis indicated that the ethanol concentration exerted an obvious effect on both DPPH free-radical scavenging potential (P<0.0001) and FRAP (P=0.0003) (Fig. 2a).
Fig. 2.
Influences of ethanol concentration (a), extraction time (b), extraction temperature (c), and solid-to-solvent ratio (d) on the DPPH free-radical scavenging potential and FRAP of phenolics from leaves of F. virens
Data are expressed as mean±SD, n=3. The same letters in the columns indicate no significant difference (Tukey’s test, P>0.05)
3.2. Effects of extraction time on EAP, EAF, and antioxidant potential
EAP increased with the increase in extraction time and reached the highest value at 50 min, ranging from (3.54±0.01)% to (4.55±0.05)% (Fig. 1b). Statistical analysis (one-way ANOVA) showed that extraction time had a significant effect (P<0.0001) on EAP. EAF values increased within the time investigated, ranging from (2.28±0.05)% to (2.99±0.02)%. However, the values of EAF obtained at 30 and 40 min were not significantly different (P>0.05). Like the EAP and EAF values, the antioxidant potential within the extraction time investigated showed that both DPPH free-radical scavenging potential and FRAP increased at longer extraction time (Fig. 2b). The highest values for both DPPH free-radical scavenging potential and FRAP were found at 50 min [(32.48±0.27) and (12.73±0.58) mg AAE/g DW, respectively]. Similar to the EAP and EAF values, extraction time was a significant variable, while DPPH free-radical scavenging potential of the extract at 20 and 30 min fell into adjacent homogeneous groups (P>0.05). Also, for both DPPH free-radical scavenging potential and FRAP, the antioxidant values of the extraction at 30, 40, and 50 min were in adjacent homogeneous groups (P>0.05). These results showed that longer extraction time did not contribute significantly to the extraction efficiency and antioxidant capacity. This might be due to some phenolic compounds being lost via oxidation and the products becoming polymerized into insoluble compounds.
3.3. Effects of temperature on EAP, EAF, and antioxidant potential
We further investigated the effect of temperature on extraction efficiency because extraction temperature impacts the solubility, mass-transfer rate, and stability of phenolic compounds (Spigno et al., 2007). The yield and antioxidant activity of natural extracts depend on the solvent and temperature used for extraction (Gironi and Piemonte, 2011). The EAF increased with increasing temperature before reaching a peak at 60 °C with a value of (2.97±0.05)%, and then decreased. Temperature effects on EAP showed a similar trend with the highest value of (4.95±0.01)% (Fig. 1c). The highest value for DPPH free-radical scavenging potential was (31.77±0.08) mg AAE/g DW, and for FRAP was (15.39±0.40) mg AAE/g DW (Fig. 2c). The results of one-way ANOVA analysis of the effects of temperature on DPPH free-radical scavenging potential and FRAP showed P values of 0.0007 and 0.0013, respectively.
The results confirmed the fact that, below a certain limit, higher temperatures improve the efficiency of extraction. This is due to enhancement of the diffusion rate and the solubility of analytes in solvents (Ju and Howard, 2003). Beyond the limit, high extraction temperatures decrease EAP and EAF. Antioxidant power, measured by DPPH free-radical scavenging potential, showed a decreasing trend with increasing temperature, which might be due to the occurrence of well known degradative phenomena (Casazza et al., 2012). Our results are in accordance with those of studies by Cacace and Mazza (2003a), Liyanapathirana and Shahidi (2005), and Pinelo et al. (2005), in which the polyphenol yield increased with increasing temperature. However, some flavonoid families are thermosensitive (Cacace and Mazza, 2003a). Therefore, the extraction temperature must be kept below a certain value.
3.4. Effects of solid-to-solvent ratio on EAP, EAF, and antioxidant potential
EAP and EAF values are presented in Fig. 1d for the five solid-to-solvent ratios tested. One-way ANOVA analysis showed that there was a significant (P<0.0001) difference among the ratios studied. This was due mainly to the 3:20 ratio (g/ml). Antioxidant potential, measured by DPPH free-radical scavenging potential and FRAP, also showed that the 3:20 ratio (g/ml) was the main contributor to the difference as measured by ANOVA (Fig. 2d). The high solubility of polyphenols in hydroalcoholic solution, especially when they are in a glycoside form, may explain the absence of variability at the higher ratios (Silva et al., 2007). According to our results, when the ratio was above 3:40 (g/ml), the quantity of phenolic compounds extracted differed only slightly. This allows the selection of any value above this limit, depending on the application of the final product. Subsequently, in our RSM design, the solid-to-solvent ratio was set at 3:40 (g/ml).
3.5. Effects of the number of extraction cycles on EAP, EAF, and antioxidant potential
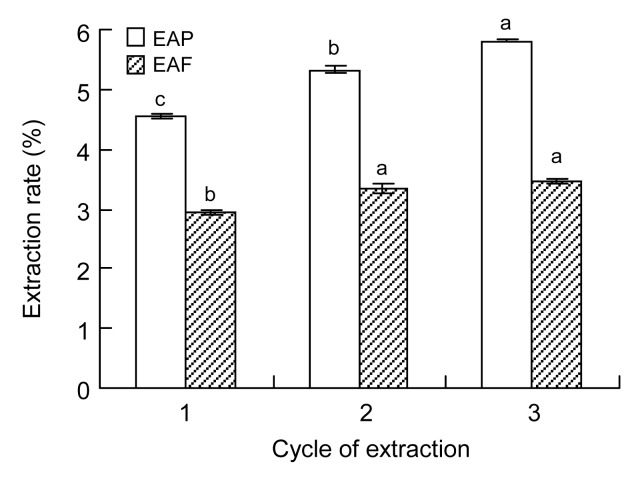
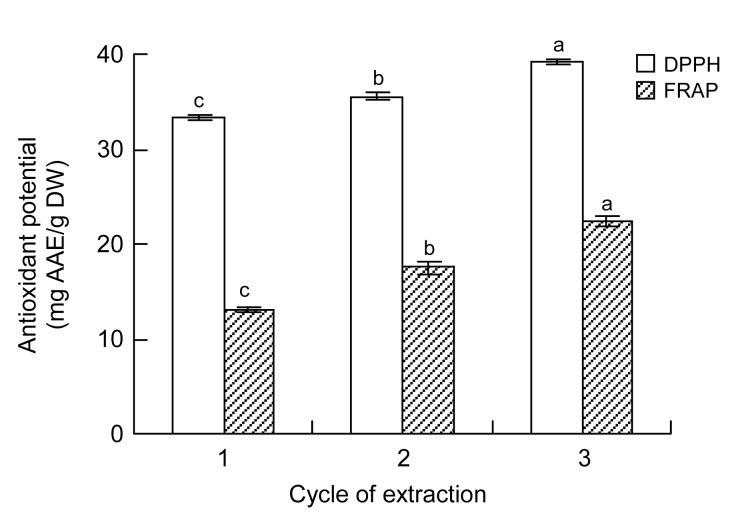
Multiple-step extraction is an important method to improve the extraction yield of polyphenols from the leaves of F. virens. To study the effect of the number of extraction cycles on the extraction ability of polyphenols, the extraction process was carried out using different numbers of cycles of extraction, and the filtrates obtained were combined. The EAP following three extraction cycles was significantly higher than that following one or two cycles (Fig. 3). The EAF following three extraction cycles was significantly (P<0.05) higher than that following a single cycle. The antioxidant potential of phenolics extracted thrice, as measured by DPPH free-radical scavenging potential and FRAP, was significantly (P<0.001) higher than that of phenolics extracted twice (Fig. 4). Based on the yield and cost, three extraction cycles were found to be most appropriate in this study.
Fig. 3.
Effects of the number of extraction cycles on EAP and EAF from leaves of F. virens
Data are expressed as mean±SD, n=3. The same letters in the columns indicate no significant difference (Tukey’s test, P>0.05)
Fig. 4.
Effects of the number of extraction cycles on the DPPH free-radical scavenging potential and FRAP of phenolics from the leaves of F. virens
Data are expressed as mean±SD, n=3. The same letters in the columns indicate no significant difference (Tukey’s test, P>0.05)
3.6. Correlations of EAP and EAF with antioxidant indices
In recent years, much research has focused on the antioxidant activity of phenolic compounds in traditional medicinal plants, and a positive correlation was observed between high phenolic content and strong antioxidant activity (Ao et al., 2008). Values of EAP and EAF were correlated with the DPPH free-radical scavenging potential and the FRAP using simple linear regression analysis. The results of our study indicated that the dependence between the variables (EAP, EAF, and antioxidant potential) showed a linear trend with good correlation coefficients (Table 3). This implies a strong positive contribution of polyphenols to antioxidant potential. The regression coefficients of DPPH (R DPPH) from high temperatures were smaller because high temperatures might cause the aggregation and degradation of phenolics (Casazza et al., 2012). The same results have been found in previous reports (Maksimović et al., 2005; Malencic et al., 2008; Abdel-Hameed, 2009). Our results also indicated that the correlation coefficients of EAP (including R DPPH and regression coefficients of FRAP (R FRAP)) and EAF (including R DPPH and R FRAP) were consistently similar. The Folin assay is related to the antioxidant potential, since oxidation of molecules leads to a lower Folin response (Huang et al., 2005). This may explain the good linear correlation between EAP and antioxidant parameters (DPPH and FRAP). Our results further confirmed the contribution of phenolics to antioxidant potential.
Table 3.
Correlation coefficients describing the relationships between EAP and EAF and the antioxidant parameters (DPPH and FRAP), determined by simple linear regression
| Independent variable | EAP |
EAF |
||
| R DPPH | R FRAP | R DPPH | R FRAP | |
| Time | 0.9244 | 0.9497 | 0.7937 | 0.9603 |
| Solid-to-solvent ratio | 0.9959 | 0.8495 | 0.9859 | 0.8367 |
| Temperature | 0.2571 | 0.8887 | 0.4381 | 0.9477 |
| Ethanol concentration | 0.7192 | 0.9366 | 0.6888 | 0.9790 |
| Number of cycles | 0.9191 | 0.9712 | 0.8110 | 0.8938 |
R DPPH and R FRAP: regression coefficients of DPPH and FRAP, respectively
3.7. Mathematical model and optimization of extraction conditions
The experiment was designed to assess the influence of four factors, i.e., ethanol concentration (%), extraction time (min), extraction temperature (°C), and the number of extraction cycles. The levels of the independent variables were chosen based on the values obtained in the single-factor experiment. The experimental values and coded levels of the four independent variables used for the 30-full factorial, central composite experimental design are given in Table 2. Values of the independent process variables considered (X 1, X 2, X 3 and X 4), as well as measured and predicted values for all responses (EAP, EAF, DPPH and FRAP), are given in Tables 4 and 5.
Table 4.
Plan and results for response surface methodology of EAP and EAF
| Run | Independent variable |
Response (%) |
||||||
| X 1 (%) | X 2 (min) | X 3 (°C) | X 4 | Observed EAP | Predicted EAP | Observed EAF | Predicted EAF | |
| 1 | 80 (+1) | 50 (+1) | 40 (−1) | 1 (−1) | 3.10 | 3.11 | 2.53 | 2.49 |
| 2 | 80 (+1) | 50 (+1) | 60 (+1) | 3 (+1) | 4.52 | 4.51 | 2.63 | 2.67 |
| 3 | 80 (+1) | 30 (−1) | 60 (+1) | 1 (−1) | 3.29 | 3.28 | 2.33 | 2.35 |
| 4 | 60 (0) | 40 (0) | 50 (0) | 2 (0) | 5.02 | 4.97 | 2.97 | 2.96 |
| 5 | 60 (0) | 40 (0) | 50 (0) | 2 (0) | 4.96 | 4.97 | 3.02 | 2.96 |
| 6 | 60 (0) | 40 (0) | 50 (0) | 2 (0) | 4.99 | 4.97 | 2.92 | 2.96 |
| 7 | 60 (0) | 40 (0) | 60 (+1) | 2 (0) | 4.91 | 4.93 | 3.23 | 3.18 |
| 8 | 40 (−1) | 30 (−1) | 40 (−1) | 1 (−1) | 3.79 | 3.79 | 2.85 | 2.81 |
| 9 | 80 (+1) | 30 (−1) | 40 (−1) | 3 (+1) | 4.07 | 4.07 | 2.12 | 2.09 |
| 10 | 60 (0) | 30 (−1) | 50 (0) | 2 (0) | 4.90 | 4.91 | 2.93 | 2.89 |
| 11 | 60 (0) | 40 (0) | 50 (0) | 1 (−1) | 4.48 | 4.49 | 2.94 | 3.01 |
| 12 | 60 (0) | 50 (+1) | 50 (0) | 2 (0) | 5.04 | 5.06 | 2.96 | 3.02 |
| 13 | 60 (0) | 40 (0) | 40 (−1) | 2 (0) | 4.49 | 4.49 | 2.75 | 2.82 |
| 14 | 40 (−1) | 50 (+1) | 40 (−1) | 1 (−1) | 4.13 | 4.12 | 2.92 | 2.91 |
| 15 | 40 (−1) | 50 (+1) | 40 (−1) | 3 (+1) | 4.93 | 4.94 | 2.56 | 2.53 |
| 16 | 80 (+1) | 50 (+1) | 40 (−1) | 3 (+1) | 4.04 | 4.04 | 2.12 | 2.14 |
| 17 | 80 (+1) | 30 (−1) | 40 (−1) | 1 (−1) | 2.60 | 2.61 | 2.11 | 2.14 |
| 18 | 80 (+1) | 50 (+1) | 60 (+1) | 1 (−1) | 3.78 | 3.79 | 2.83 | 2.80 |
| 19 | 60 (0) | 40 (0) | 50 (0) | 2 (0) | 5.02 | 4.97 | 2.93 | 2.96 |
| 20 | 40 (−1) | 30 (−1) | 60 (+1) | 1 (−1) | 4.19 | 4.19 | 3.04 | 3.01 |
| 21 | 40 (−1) | 30 (−1) | 40 (−1) | 3 (+1) | 5.16 | 5.15 | 2.71 | 2.73 |
| 22 | 40 (−1) | 30 (−1) | 60 (+1) | 3 (+1) | 5.35 | 5.35 | 3.11 | 3.15 |
| 23 | 40 (−1) | 50 (+1) | 60 (+1) | 3 (+1) | 5.16 | 5.15 | 3.08 | 3.04 |
| 24 | 80 (+1) | 30 (−1) | 60 (+1) | 3 (+1) | 4.54 | 4.54 | 2.52 | 2.53 |
| 25 | 60 (0) | 40 (0) | 50 (0) | 2 (0) | 4.94 | 4.97 | 3.01 | 2.96 |
| 26 | 60 (0) | 40 (0) | 50 (0) | 3 (+1) | 5.50 | 5.53 | 2.96 | 2.91 |
| 27 | 80 (+1) | 40 (0) | 50 (0) | 2 (0) | 3.95 | 3.95 | 2.38 | 2.35 |
| 28 | 60 (0) | 40 (0) | 50 (0) | 2 (0) | 4.94 | 4.97 | 2.96 | 2.96 |
| 29 | 40 (−1) | 40 (0) | 50 (0) | 2 (0) | 4.82 | 4.86 | 2.83 | 2.88 |
| 30 | 40 (−1) | 50 (+1) | 60 (+1) | 1 (−1) | 4.55 | 4.54 | 3.18 | 3.21 |
X 1: ethanol concentration; X 2: extraction time; X 3: extraction temperature; X 4: number of extraction cycles. The coded forms of the variables are shown in parentheses
Table 5.
Plan and results for response surface methodology of DPPH free-radical scavenging potential and FRAP
| Run | Independent variable |
Response (mg AAE/g DW) |
||||||
| X 1 (%) | X 2 (min) | X 3 (°C) | X 4 | Observed DPPH | Predicted DPPH | Observed FRAP | Predicted FRAP | |
| 1 | 80 (+1) | 50 (+1) | 40 (−1) | 1 (−1) | 33.60 | 33.03 | 10.09 | 9.92 |
| 2 | 80 (+1) | 50 (+1) | 60 (+1) | 3 (+1) | 46.90 | 47.07 | 14.47 | 14.60 |
| 3 | 80 (+1) | 30 (−1) | 60 (+1) | 1 (−1) | 29.86 | 29.07 | 10.62 | 10.79 |
| 4 | 60 (0) | 40 (0) | 50 (0) | 2 (0) | 53.84 | 55.10 | 15.86 | 15.74 |
| 5 | 60 (0) | 40 (0) | 50 (0) | 2 (0) | 53.44 | 55.10 | 15.37 | 15.74 |
| 6 | 60 (0) | 40 (0) | 50 (0) | 2 (0) | 57.01 | 55.10 | 15.95 | 15.74 |
| 7 | 60 (0) | 40 (0) | 60 (+1) | 2 (0) | 56.14 | 55.29 | 15.78 | 15.66 |
| 8 | 40 (−1) | 30 (−1) | 40 (−1) | 1 (−1) | 32.46 | 32.27 | 11.90 | 11.73 |
| 9 | 80 (+1) | 30 (−1) | 40 (−1) | 3 (+1) | 38.92 | 38.14 | 13.25 | 13.16 |
| 10 | 60 (0) | 30 (−1) | 50 (0) | 2 (0) | 53.74 | 54.50 | 15.50 | 15.42 |
| 11 | 60 (0) | 40 (0) | 50 (0) | 1 (−1) | 44.34 | 45.25 | 14.40 | 14.57 |
| 12 | 60 (0) | 50 (+1) | 50 (0) | 2 (0) | 56.60 | 55.62 | 15.13 | 15.37 |
| 13 | 60 (0) | 40 (0) | 40 (−1) | 2 (0) | 50.24 | 50.86 | 13.67 | 13.95 |
| 14 | 40 (−1) | 50 (+1) | 40 (−1) | 1 (−1) | 33.31 | 32.88 | 11.69 | 11.83 |
| 15 | 40 (−1) | 50 (+1) | 40 (−1) | 3 (+1) | 40.03 | 40.79 | 13.53 | 13.33 |
| 16 | 80 (+1) | 50 (+1) | 40 (−1) | 3 (+1) | 38.94 | 39.30 | 12.73 | 12.75 |
| 17 | 80 (+1) | 30 (−1) | 40 (−1) | 1 (−1) | 28.25 | 28.63 | 9.33 | 9.34 |
| 18 | 80 (+1) | 50 (+1) | 60 (+1) | 1 (−1) | 33.70 | 33.93 | 11.77 | 11.59 |
| 19 | 60 (0) | 40 (0) | 50 (0) | 2 (0) | 55.95 | 55.10 | 15.68 | 15.74 |
| 20 | 40 (−1) | 30 (−1) | 60 (+1) | 1 (−1) | 33.64 | 33.36 | 13.34 | 13.31 |
| 21 | 40 (−1) | 30 (−1) | 40 (−1) | 3 (+1) | 43.57 | 43.42 | 14.05 | 14.22 |
| 22 | 40 (−1) | 30 (−1) | 60 (+1) | 3 (+1) | 50.83 | 51.37 | 15.84 | 15.98 |
| 23 | 40 (−1) | 50 (+1) | 60 (+1) | 3 (+1) | 49.49 | 49.21 | 15.32 | 15.30 |
| 24 | 80 (+1) | 30 (−1) | 60 (+1) | 3 (+1) | 44.93 | 45.45 | 14.94 | 14.80 |
| 25 | 60 (0) | 40 (0) | 50 (0) | 2 (0) | 54.56 | 55.10 | 16.18 | 15.74 |
| 26 | 60 (0) | 40 (0) | 50 (0) | 3 (+1) | 58.52 | 57.39 | 17.33 | 17.32 |
| 27 | 80 (+1) | 40 (0) | 50 (0) | 2 (0) | 42.20 | 42.68 | 12.93 | 13.19 |
| 28 | 60 (0) | 40 (0) | 50 (0) | 2 (0) | 55.17 | 55.10 | 15.85 | 15.74 |
| 29 | 40 (−1) | 40 (0) | 50 (0) | 2 (0) | 46.28 | 45.57 | 14.84 | 14.74 |
| 30 | 40 (−1) | 50 (+1) | 60 (+1) | 1 (−1) | 33.69 | 34.43 | 13.56 | 13.62 |
X 1: ethanol concentration; X 2: extraction time; X 3: extraction temperature; X 4: number of extraction cycles. The coded forms of the variables are shown in parentheses
The experimental values of all indices were analyzed by multiple regression to fit the second-order polynomial equations shown in Table 6. The quality of fit was ascertained using the coefficients of determination (R 2). The experimental data obtained showed a good fit with the equations (P<0.0001). This indicated an excellent agreement between observed and predicted responses and that the derived equations could adequately predict the experimental results. The utilization of the predictive models enabled the theoretical calculation of the ideal sets of conditions under which maximal values could be attained (Table 7). The ideal extraction conditions obtained for EAP, EAF, DPPH free-radical scavenging potential, and FRAP varied. The ideal ethanol concentrations for EAP, EAF, and DPPH free-radical scavenging potential were lower than that for FRAP. The ideal extraction temperature was 53 °C for maximum EAP, 60 °C for maximum EAF, 59 °C for DPPH free-radical scavenging potential, and 55 °C for FRAP. The ideal extraction time was 30 min for maximum EAP, 50 min for EAF, 37 min for DPPH free-radical scavenging potential, and 38 min for FRAP. The ideal number of extraction cycles was three for EAP and one for EAF, and three for DPPH free-radical scavenging potential and FRAP. The differences in the ideal extraction temperature and extraction time for maximum EAP and EAF could be due to the stability of the phenolic compounds being affected by chemical and enzymatic degradation, and losses by volatilization or other thermal decomposition (Juntachote et al., 2006). Both DPPH free-radical scavenging potential and FRAP gave accurate, repeatable values, but the values for antioxidant potential given by these two methods were significantly different. From a mechanical standpoint, in FRAP there is a single-electron transfer reaction, while in DPPH there are both single-electron transfers and hydrogen atom transfer reactions (Pérez-Jiménez et al., 2008). This difference might result in different antioxidant potentials and ideal ethanol concentrations being obtained.
Table 6.
Polynomial equations and statistical parameters describing the effects of the independent variables considered on EAP, EAF, and the antioxidant potential measured by DPPH free-radical scavenging potential and FRAP, calculated after implementation of a 30-full factorial, central composite experimental design
| Response | Second-order polynomial equation | R 2 | P |
| EAP | 4.97−0.46X 1+0.076X 2+0.22X 3+0.52X 4+0.041X 1 X 2+0.068X 1 X 3+0.028X 1 X 4+0.00248X 2 X 3−0.14X 2 X 4−0.051X 3 X 4−0.57X 1 2+0.015X 2 2−0.26X 3 2+0.038X 4 2 | 0.9992 | <0.0001 |
| EAF | 2.96−0.26X 1+0.062X 2+0.18X 3−0.051X 4+0.063X 1 X 2+0.004131X 1 X 3+0.008234X 1 X 4+0.023X 2 X 3−0.075X 2 X 4−0.054X 3 X 4−0.34X 1 2−0.001526X 2 2+0.045X 3 2+0.005301X 4 2 | 0.9852 | <0.0001 |
| DPPH | 55.10332−1.44464X 1+0.558671X 2+2.214841X 3+6.072034X 4+0.947847X 1 X 2−0.16304X 1 X 3−0.40907X 1 X 4+0.115876X 2 X 3−0.81115X 2 X 4+1.715618X 3 X 4−10.9751X 1 2−0.04567X 2 2−2.02506X 3 2−3.78615X 4 2 | 0.9929 | <0.0001 |
| FRAP | 15.73672−0.77406X 1−0.02637X 2+0.856915X 3+1.37589X 4+0.121812X 1 X 2−0.03062X 1 X 3+0.332969X 1 X 4+0.053478X 2 X 3−0.2474X 2 X 4+0.045085X 3 X 4−1.77457X 1 2−0.34329X 2 2−0.93265X 3 2−0.205364X 4 2 | 0.9916 | <0.0001 |
X 1: ethanol concentration; X 2: extraction time; X 3: extraction temperature; X 4: number of extraction cycles
Table 7.
Ideal predicted conditions and theoretically calculated maximal values for EAP, EAF and antioxidant potential
| Response | Maximum predicted value | Ideal condition |
|||
| Ethanol (%) | Time (min) | Temperature (°C) | Number of cycles | ||
| EAP | 5.72% | 52 | 30 | 53 | 3 |
| EAF | 3.09% | 59 | 50 | 60 | 1 |
| DPPH | 58.88 mg AAE/g DW | 54 | 37 | 59 | 3 |
| FRAP | 15.86 mg AAE/g DW | 77 | 38 | 55 | 3 |
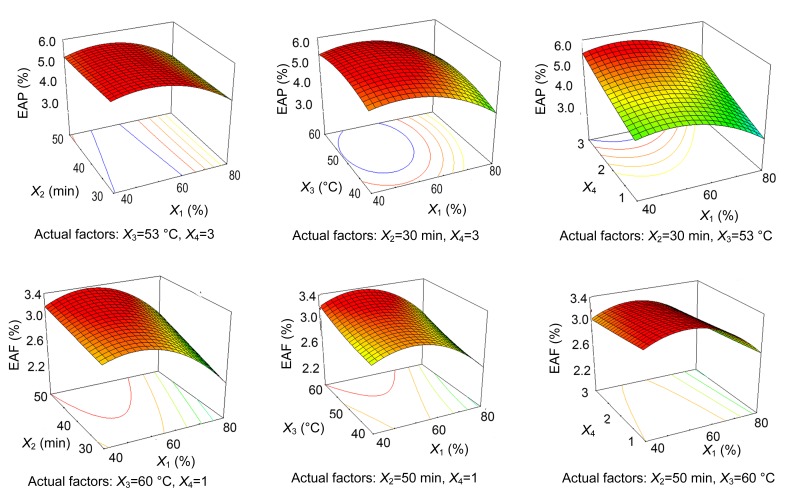
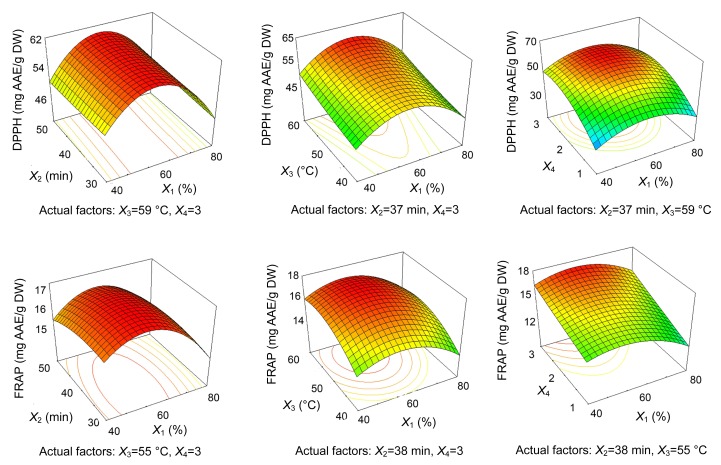
The trends revealed in each response were recorded in the form of three-dimensional plots (Figs. 5 and 6), which show the effect of simultaneous variation in ethanol concentration and extraction time, extraction temperature, and the number of extraction cycles.
Fig. 5.
Tri-dimensional response surface contour plots showing the effect of co-variance in ethanol concentration (X 1)/extraction time (X 2) (left), ethanol concentration (X 1)/extraction temperature (X 3) (middle), and ethanol concentration (X 1)/number of cycles (X 4) (right) on the EAP and EAF from leaves of F. virens
Fig. 6.
Tri-dimensional response surface contour plots showing the effect of co-variance in ethanol concentration (X 1)/extraction time (X 2) (left), ethanol concentration (X 1)/extraction temperature (X 3) (middle), and ethanol concentration (X 1)/number of cycles (X 4) (right) on the DPPH free-radical scavenging potential and FRAP of polyphenols from leaves of F. virens
The trend for EAP from F. virens leaves upon simultaneous variation of ethanol concentration with extraction time, extraction temperature, and number of extraction cycles is shown in Fig. 5. The maximum EAP can be achieved at an intermediate ethanol concentration and a low time value. At higher ethanol concentrations and longer extraction time, EAP showed a declining tendency. When the extraction time was set at 30 min, EAP increased with ethanol concentration up to a maximum at about 52% ethanol, 53 °C, and three extraction cycles, and then decreased with further increases in ethanol concentration.
Higher EAF values were obtained when the number of extraction cycles was set at one, ethanol concentration at intermediate levels, extraction time at 50 min, and temperature at higher values. Similar to the conditions obtained for EAP, an intermediate level of ethanol concentration resulted in a good extraction efficiency. However, compared with EAP, the ideal EAF was achieved at a higher temperature.
The DPPH free-radical scavenging potential was affected mainly by the number of extraction cycles. DPPH free-radical scavenging potential increased with ethanol concentration up to a maximum at about 54% and then decreased with further increases in ethanol concentration. At the same time, longer extraction time, higher extraction temperature, and more extraction cycles resulted in higher antioxidant potential.
With regard to FRAP, intermediate ethanol concentrations and temperatures were adequate to achieve high values, which were in accordance with the values obtained for DPPH free-radical scavenging potential. Lower extraction time and more cycles of extraction sufficed to obtain strong antioxidant potential.
To reach a consensus on an ideal extraction condition, four sets of conditions obtained for EAP, EAF, DPPH free-radical scavenging potential, and FRAP were assigned weights of 2, 1, 2, and 1, respectively. The weighted average was assumed to be the ideal extraction condition. The assignment of weights was based on the following assumption: flavonoids are one class of phenolics which have ketone-containing compounds. We assigned more weight to phenolics which represent the profile of phenolic compounds extracted more generally. Similarly, the mechanism of DPPH free-radical scavenging assay consists of a single-electron transfer and a hydrogen atom transfer reaction, while in FRAP there is only a single-electron transfer reaction (Pérez-Jiménez et al., 2008). So the ideal condition for higher DPPH free-radical scavenging potential was given more weight. The ideal extraction condition thus obtained was 50% ethanol, 37 min, 57 °C, with three extraction cycles.
4. Conclusions
Leaves of F. virens are rich in phenolic compounds which show strong antioxidant potential as measured by four indices: EAP, EAF, DPPH free-radical scavenging potential, and FRAP. From the results of single-factor experiments, we concluded that the five parameters studied (ethanol concentration, extraction time, extraction temperature, solid-to-solvent ratio, and the number of extraction cycles) all had significant effects on the extraction rates of phenolics and flavonoids from the leaves of F. virens, and on the antioxidant potential of the extracts. The ideal conditions for the extractions of phenolics, flavonoids, and antioxidants from F. virens leaves were studied using RSM. Following mathematical optimization analysis, the ideal conditions were obtained. Furthermore, high correlations between EAP and EAF and antioxidant potential confirmed that phenolic compounds contribute to the antioxidant potential. In conclusion, the ethanol extract from leaves of F. virens exhibited a high level of antioxidant capacity, suggesting that such extracts are an ideal candidate for product developers to address the health effects of oxidative stress. The results of this report could contribute to enhancing the utilization of leaf extracts of F. virens in food, pharmaceutical, and cosmetic industries.
Footnotes
Project supported by the National Natural Science Foundation of China (No. 31070522) and the Science and Technology Foundation of Fujian Province (No. 2010N5013), China
Compliance with ethics guidelines: Xiao-xin CHEN, Xiao-bing WU, Wei-ming CHAI, Hui-ling FENG, Yan SHI, Han-tao ZHOU, and Qing-xi CHEN declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Abdel-Hameed ESS. Total phenolic contents and free radical scavenging activity of certain Egyptian Ficus species leaf samples. Food Chem. 2009;114(4):1271–1277. doi: 10.1016/j.foodchem.2008.11.005. [DOI] [Google Scholar]
- 2.Ao C, Li A, Elzaawely AA, Xuan TD, Tawata S. Evaluation of antioxidant and antibacterial activities of Ficus microcarpa L. fil. extract. Food Control. 2008;19(10):940–948. doi: 10.1016/j.foodcont.2007.09.007. [DOI] [Google Scholar]
- 3.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 4.Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol. 1995;28(1):25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- 5.Bucić-Kojić A, Planinić M, Tomas S, Jakobek L, Šeruga M. Influence of solvent and temperature on extraction of phenolic compounds from grape seed, antioxidant activity and colour of extract. Int J Food Sci Technol. 2009;44(12):2394–2401. doi: 10.1111/j.1365-2621.2008.01876.x. [DOI] [Google Scholar]
- 6.Cacace JE, Mazza G. Mass transfer process during extraction of phenolic compounds from milled berries. J Food Eng. 2003;59(4):379–389. doi: 10.1016/S0260-8774(02)00497-1. [DOI] [Google Scholar]
- 7.Cacace JE, Mazza G. Optimization of extraction of anthocyanins from black currants with aqueous ethanol. J Food Sci. 2003;68(1):240–248. doi: 10.1111/j.1365-2621.2003.tb14146.x. [DOI] [Google Scholar]
- 8.Cai Y, Luo Q, Sun M, Harold C. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004;74(17):2157–2184. doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casazza AA, Aliakbarian B, Sannita E, Perego P. High-pressure high-temperature extraction of phenolic compounds from grape skins. Int J Food Sci Technol. 2012;47(2):399–405. doi: 10.1111/j.1365-2621.2011.02853.x. [DOI] [Google Scholar]
- 10.Contini M, Baccelloni S, Massantini R, Anelli G. Extraction of natural antioxidants from hazelnut (Corylus avellana L.) shell and skin wastes by long maceration at room temperature. Food Chem. 2008;110(3):659–669. doi: 10.1016/j.foodchem.2008.02.060. [DOI] [Google Scholar]
- 11.Dixon RA, Paiva NL. Stress-induced phenylpropanoid metabolism. Plant Cell. 1995;7(7):1085–1097. doi: 10.1105/tpc.7.7.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gironi F, Piemonte V. Temperature and solvent effects on polyphenol extraction process from chestnut tree wood. Chem Eng Res Des. 2011;89(7A):857–862. doi: 10.1016/j.cherd.2010.11.003. [DOI] [Google Scholar]
- 13.Huang D, Ou B, Prior RL. The chemistry behind antioxidant capacity assays. J Agric Food Chem. 2005;53(6):1841–1856. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- 14.Jia Z, Tang M, Wu J. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64(4):555–559. doi: 10.1016/S0308-8146(98)00102-2. [DOI] [Google Scholar]
- 15.Ju ZY, Howard LR. Effects of solvent and temperature on pressurized liquid extraction of anthocyanins and total phenolics from dried red grape skin. J Agric Food Chem. 2003;51(18):5207–5213. doi: 10.1021/jf0302106. [DOI] [PubMed] [Google Scholar]
- 16.Juntachote T, Berghofer E, Bauer F, Siebenhandl S. The application of response surface methodology to the production of phenolic extracts of lemon grass, galangal, holy basil and rosemary. Int J Food Sci Tech. 2006;41(2):121–133. doi: 10.1111/j.1365-2621.2005.00987.x. [DOI] [Google Scholar]
- 17.Khan KY, Khan MA, Niamat R, Munir M, Fazal H, Mazari P, Seema N, Bashir T, Kanwal A, Ahmed SN. Element content analysis of plants of genus Ficus using atomic absorption spectrometer. Afr J Pharm Pharmacol. 2011;5(3):317–321. doi: 10.5897/AJPP10.339. [DOI] [Google Scholar]
- 18.Liyanapathirana C, Shahidi F. Optimization of extraction of phenolic compounds from wheat using response surface methodology. Food Chem. 2005;93(1):47–56. doi: 10.1016/j.foodchem.2004.08.050. [DOI] [Google Scholar]
- 19.Maksimović Z, Malenčić D, Kovačević N. Polyphenol contents and antioxidant activity of Maydis stigma extracts. Bioresour Technol. 2005;96(8):873–877. doi: 10.1016/j.biortech.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Malencic D, Maksimovic Z, Popovic M, Miladinovic J. Polyphenol contents and antioxidant activity of soybean seed extracts. Bioresour Technol. 2008;99(14):6688–6691. doi: 10.1016/j.biortech.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 21.Pérez-Jiménez J, Arranz S, Tabernero M, Elena Díaz-Rubio M, Serrano J, Goñi I, Saura-Calixto F. Updated methodology to determine antioxidant capacity in plant foods, oils and beverages: extraction, measurement and expression of results. Food Res Int. 2008;41(3):274–285. doi: 10.1016/j.foodres.2007.12.004. [DOI] [Google Scholar]
- 22.Pinelo M, Rubilar M, Jerez M, Sineiro J, Nuanez MJ. Effect of solvent, temperature, and solvent-to-solid ratio on the total phenolic content and antiradical activity of extracts from different components of grape pomace. J Agric Food Chem. 2005;53(6):2111–2117. doi: 10.1021/jf0488110. [DOI] [PubMed] [Google Scholar]
- 23.Sasaki YF, Kawaguchi S, Kamay A, Ohshit M, Kabasawa K, Iwama K, Taniguchi K, Tsuda S. The comet assay with 8 mouse organs: results with 39 currently used food additives. Mutat Res. 2002;519(1-2):103–119. doi: 10.1016/S1383-5718(02)00128-6. [DOI] [PubMed] [Google Scholar]
- 24.Shi YX, Xu YK, Hu HB, Na Z, Wang WH. Preliminary assessment of antioxidant activity of young edible leaves of seven Ficus species in the ethnic diet in Xishuangbanna, Southwest China. Food Chem. 2011;128(4):889–894. doi: 10.1016/j.foodchem.2011.03.113. [DOI] [Google Scholar]
- 25.Silva EM, Rogez H, Larondelle Y. Optimization of extraction of phenolics from Inga edulis leaves using response surface methodology. Sep Purif Technol. 2007;55(3):381–387. doi: 10.1016/j.seppur.2007.01.008. [DOI] [Google Scholar]
- 26.Spigno G, Tramelli L, Faveri DM. Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. J Food Eng. 2007;81(1):200–208. doi: 10.1016/j.jfoodeng.2006.10.021. [DOI] [Google Scholar]
- 27.Thaipong K, Boonprakob U, Crosby K, Cisneros-Zevallos L, Byrne DH. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J Food Compos Anal. 2006;19(6-7):669–675. doi: 10.1016/j.jfca.2006.01.003. [DOI] [Google Scholar]
- 28.Thoo YY, Ho SK, Liang JY, Ho CW, Tan CP. Effects of binary solvent extraction system, extraction time and extraction temperature on phenolic antioxidants and antioxidant capacity from mengkudu (Morinda citrifolia) Food Chem. 2010;120(1):290–295. doi: 10.1016/j.foodchem.2009.09.064. [DOI] [Google Scholar]
- 29.Wei SD, Zhou HC, Lin YM. Antioxidant activities of extract and fractions from the hypocotyls of the mangrove plant Kandelia candel . Int J Mol Sci. 2010;11(10):4080–4093. doi: 10.3390/ijms11104080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu X, Yu X, Jing H. Optimization of phenolic antioxidant extraction from Wuweizi (Schisandra chinensis) pulp using random-centroid optimazation methodology. Int J Mol Sci. 2011;12(12):6255–6266. doi: 10.3390/ijms12096255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang L, Jiang JG, Li WF, Chen J, Wang DY, Zhu L. Optimum extraction process of polyphenols from the bark of Phyllanthus emblica L. based on the response surface methodology. J Sep Sci. 2009;32(9):1437–1444. doi: 10.1002/jssc.200800744. [DOI] [PubMed] [Google Scholar]
- 32.Zhang ZS, Li D, Wang LJ, Ozkan N, Chen XD, Mao ZH, Yang HZ. Optimization of ethanol-water extraction of lignans from flaxseed. Sep Purif Technol. 2007;57(1):17–24. doi: 10.1016/j.seppur.2007.03.006. [DOI] [Google Scholar]