Lay summary
Experiments with foraging house sparrows show that chance events during learning can explain contrasting individual preferences for safe versus risky alternatives, even when the expected benefit of the risky alternativeis 8-fold higher than that of the safe one. However, in social groups, learning to prefer the safe but less rewarding alternative mayoccasionally be compensated by scrounging on the food findings of individuals that havelearned to prefer the high risk-high reward option.
Key words: decision making, producer, risk sensitivity, scrounger, social foraging, social learning.
Abstract
Although there has been extensive research on the evolution of individual decision making under risk (when facing variable outcomes), little is known on how the evolution of such decision-making mechanisms has been shaped by social learning and exploitation. We presented socially foraging house sparrows with a choice between scattered feeding wells in which millet seeds were hidden under 2 types of colored sand: green sand offering ~80 seeds with a probability of 0.1 (high risk–high reward) and yellow sand offering 1 seed with certainty (low risk–low reward). Although the expected benefit of choosing variable wells was 8 times higher than that of choosing constant wells, only some sparrows developed a preference for variable wells, whereas others developed a significant preference for constant wells. We found that this dichotomy could be explained by stochastic individual differences in sampling success during foraging, rather than by social foraging strategies (active searching vs. joining others). Moreover, preference for variable or constant wells was related to the sparrows’ success during searching, rather than during joining others or when picking exposed seeds (i.e., they learn when actively searching in the sand). Finally, although for many sparrows learning resulted in an apparently maladaptive risk aversion, group living still allowed them to enjoy profitable variable wells by occasionally joining variable-preferring sparrows.
INTRODUCTION
Extensive research on decision making in humans and animals has focused on choices between safe and variable outcomes (e.g., Kahneman and Tversky 1979; Caraco et al. 1980; Stephens 1981; Kacelnik and Bateson 1996, 1997; Weber et al. 2004; Wu and Giraldeau 2005). In this context, the term “risk” refers to variability in reward and the term “risk sensitivity” refers to how decision makers respond to such variability. Although most earlier attempts to explain decision making under risk were based on normative models, such as those relating risk seeking or risk aversion to decelerating or accelerating utility functions (Kahneman and Tversky 1979; McNamara and Houston 1992), recent efforts have mainly focused on process-based models, which consider the mechanisms by which experience is coded and used for making further choices (e.g., March 1996; Kacelnik and Bateson 1997; Erev and Barron 2005; Shafir et al. 2008). A particular set of cases where process-based models can explain phenomena such as risk aversion and underweighting of rare events is represented in reinforcement learning models (March 1996; Niv et al. 2002; Hertwig et al. 2004). For example, it has been shown that the tendency to stop exploring options that are unsuccessful (the “hot-stove effect”; Denrell and March 2001) can gradually lead to risk aversion (March 1996) and that under some conditions, learning rules may produce apparently maladaptive preference for a more assured but less profitable alternative (Erev and Barron 2005; Arbilly et al. 2010).
How learning or cognitive mechanisms that produce suboptimal behavior evolve is a challenging problem. It is increasingly acknowledged that to answer this question, we need to focus on the evolution of explicit learning rules (McNamara and Houston 2009; Fawcett et al. 2012). Early work along this line suggested that a trade-off between exploration and exploitation may provide a good answer in some cases (Real 1991; Keasar 2002; Niv et al. 2002), but not in others (Kacelnik and Bateson 1996). Another direction has focused on general cognitive constraints, such as those resulting from Weber’s law or from fundamental principles of associative learning (Kacelnik and Brito e Abreu 1998; Weber et al. 2004; Trimmer et al. 2012). These cognitive principles may prevent optimization for a particular task (or experimental test) but could have evolved to serve a much broader set of behaviors on a much larger scale of ecological conditions (see discussions by Weber et al. 2004; Trimmer et al. 2012). An alternative and more modest approach is to examine learning and decision-making mechanisms on just a slightly broader ecological scale (see e.g., Todd and Gigerenzer 2000; Stephens et al. 2004). Most of the above-mentioned research has been focused on isolated individuals making decisions under variable conditions without interacting with one another. As a result, the interaction between the learning dynamics and social foraging or social learning has received relatively little attention. An intriguing possibility, therefore, is that some of the apparently maladaptive outcomes of learning may be better understood when the social context, under which they are likely to operate in nature, is also considered.
In the field of social foraging, where individuals are engaged in a frequency-dependent game between producers and scroungers (Barnard and Sibly 1981; Giraldeau and Caraco 2000; Giraldeau and Dubois 2008), explicit modeling of learning mechanisms or experimental studies of learning have just started to emerge. Most of these studies are focused on learning to choose between producer and scrounger strategies (Beauchamp 2000; Katsnelson et al. 2008; Hamblin and Giraldeau 2009; Dubois et al. 2010; Morand-Ferron and Giraldeau 2010; Belmaker et al. 2012; Katsnelson et al. 2012), but a few theoretical studies have explored how learning to find food in a variable environment may be affected by the social context of the producer–scrounger game (Arbilly et al. 2010, 2011). Previously, it has been suggested that group foraging is less risky (Caraco 1981) and that risk-averse individuals should scrounge more (Caraco 1981; Lendvai et al. 2004) or less (Morgan et al. 2011), but the role of learning in mediating such decisions is unclear. Similarly, extensive theoretical research on the evolution of social learning has focused on the adaptive value of information transfer and on the conditions for its success (e.g., Boyd and Richerson 1985; Feldman et al. 1996; Dall et al. 2005; Galef and Laland 2005), but only recently has the evolution of explicit rules for social learning been investigated (Rendell et al. 2010).
In the context of the producer–scrounger game, it has been suggested that scroungers (that join other group members that find food) are potentially social learners (Giraldeau 1997; Laland 2004). It has also been noted that producing is usually a high-risk strategy, whereas scrounging on individuals that have already found food is expected to result in lower variability in intake rates (Wu and Giraldeau 2005). Accordingly, we hypothesized that it might be easier for scroungers to learn to prefer patches associated with rare but highly profitable food (high risk–high reward) than for producers. This is because producers are likely to be discouraged by repeated failures in empty patches of this type, whereas scroungers follow others that have already found food in this type of patch and are therefore much more likely to experience success. In other words, scroungers have more opportunities to learn to prefer the patches that provide rare but highly profitable reward. This point was demonstrated in a recent theoretical analysis (Arbilly et al. 2011), which found that when high expected payoffs are associated with a high risk of failure, social learning is increasingly adaptive; in fact, social learning can circumvent the problem of risk aversion that may be developed by individual learners. It is not clear, though, that all social foragers use social learning. In some cases, there is evidence that scrounging can actually interfere with learning of food-related cues (Giraldeau and Lefebvre 1987; Beauchamp and Kacelnik 1991). Nevertheless, group foraging may still compensate for maladaptive risk aversion if some lucky individuals find rare resources and others simply join them.
In this study, we examine how individual and social experiences affect risk-related decision making in socially foraging house sparrows (Passer domesticus). We presented socially foraging house sparrows with a grid of food-containing wells. The content of the wells was hidden under either green- or yellow-colored sand: 10% of the green wells contained 80 seeds and 90% contained no seeds (high risk–high reward, hereafter “variable wells”), whereas all yellow wells (100%) contained 1 seed with certainty (low risk–low reward, hereafter “constant wells”). Although the expected benefit of choosing variable (green) wells was 8 times higher than that of choosing constant (yellow) wells, in practice, due to the process of sampling, there was a higher chance that the birds would initially experience frequent failures in variable wells and frequent success in constant wells. We tested whether sparrows can learn to prefer the variable risky patches and how this preference might be explained by their individual experience, social foraging strategy, or social experience (when following others). As explained above, we hypothesized that it might be easier for scroungers to learn to prefer patches associated with rare but highly profitable food (i.e., the variable green patches) than for producers. We also tried to assess the extent to which learning and its behavioral consequences become adaptive, given the sparrows’ social lifestyle.
METHODS
Study animals and experimental setup
During the winter of 2009, sixty wild house sparrows (P. domesticus) were caught within the I. Meier Segals Garden for Zoological Research, Tel-Aviv University, Israel. House sparrows are extremely abundant and legally unprotected in Israel. The experiments were conducted under a permit from Tel-Aviv University Animal Care Committee (L-09-015). The birds were divided into 6 flocks of 5 males and 5 females. Each flock was housed separately in an outdoor aviary (3.9×3.9×3.6 m). A wooden grid (130×130cm) containing 100 wells (2.5cm in diameter, 1cm depth, and 7.5cm center to center) was positioned on the ground at the center of the aviary. When not participating in an experiment, the birds were given food (a mixture of commercial bird feed, insects, and grated boiled egg) and water ad libitum.
To allow individual identification from a top view angle (see The experimental procedure), the 2 central tail rectrices were cut near their base and 2 colored feathers of similar size were glued to the original feather base (following the technique used by Bro-Jørgensen et al. 2007 to manipulate tail length in barn swallows).
To facilitate habituation to the experimental setup, the birds’ food was initially placed on the surface of the wooden grid. Gradually, millet seeds were put in the wells and were covered with uncolored sand, and the birds practiced searching for 10–15 seeds hidden in the sand. During the 8 days that preceded the experiment, the birds participated in another experiment that involved searching for seeds covered with red or blue sand (Ilan 2011). All birds went through the same procedure and it is unlikely that this previous experience affected our main results (see Discussion).
All birds survived in good condition (based on physical examination of plumage and body mass). Following the experiment, 30 sparrows were released in the area of capture (see release procedure in Katsnelson et al. 2008, mean ± standard deviation body mass of released birds was 27.61±1.9g) and 30 were kept as a part of a breeding colony in the I. Meier Segals Zoological Garden (see Dor and Lotem 2009).
The experimental procedure
The experiment spanned 2 days and included a training phase and a test phase as detailed below. Birds were food deprived overnight before each experimental day. An experimental day started 2.5h after sunrise. In both phases, birds’ foraging behavior was recorded by a digital video camera located above the grid (top view).
The training phase
This phase included 5 trials: 4 trials in intervals of 10–15min were conducted on the first day and a fifth trial was conducted on the morning of the second day. Trial duration was 2min from the time the first bird landed on the grid.
In each trial, 50 wells were randomly assigned to “constant condition” and 50 to “variable condition.” In the constant condition, a single millet seed was placed in each of the 50 wells and covered with yellow-colored sand. Hence, the yellow sand is a cue for constant (low risk–low reward) payoff. In the variable condition, 80 millet seeds were placed in only 5 randomly assigned wells out of the 50 (hereafter referred to as “profitable variable wells”), and all 50 wells were covered with green-colored sand. Hence, the green sand is a cue for variable (high risk–high reward) payoff. In both conditions, we used the smallest amount of sand required to conceal the seeds, thus, ensuring that seeds would easily be obtained by pecking the sand. The birds could not see the placement of seeds by the experimenter because wells had inner cups that were prepared in advance in our laboratory (arranged on a tray) and placed carefully in each designated well after the seeds were already covered with sand. Note that the expected value of the variable wells is 8 times higher than that of the constant wells (80 with a probability of 0.1 vs. 1 with certainty) and that even when competition is considered, it would be predicted that most individuals should prefer the variable patch (possibly in a ratio of 8:1, if adopting the concept of Ideal Free Distribution by Fretwell and Lucas (1968) as a null model). It is also important to note that although each condition is characterized by 1 color (i.e., green is always variable and yellow is always constant), all individuals were exposed to these conditions of variable (green) and constant (yellow) wells. Thus, analyzing differences between individuals tested under the same conditions (which is the focus of the present study) is unlikely to be confounded by color (see Discussion).
The test phase
Following the last training phase (in the morning of the second day), the test phase was conducted. The aim of this phase was to test whether birds had developed a preference for the variable wells (green sand) or for the constant wells (yellow sand) after their experience during the training phase. As in the training phase, wells were randomly assigned to be filled with green or yellow sand (50 of each), corresponding to previously variable and constant wells. However, all wells in the grid contained no food but only colored sand during this phase (i.e., subjects were tested “in extinction”—a common procedure that cannot bias previously learned preferences but can gradually extinguish them).
Behavioral and data analysis
The colored tail feathers (see Study animals and experimental setup) allowed individual identification during video analysis. We followed each bird separately (focal analysis) from the time it landed on the grid until it left. We included in our analysis only birds that preformed at least 6 foraging steps during both the training phase and the test phase (see details below). A foraging step was defined as a visit to a well that included at least 1 peck by the focal bird. The 6-step criterion was chosen a priori because this would be the minimal number of steps that allows a significant preference to be detected (using a sign test). We also analyzed the data of all birds that could be identified in both experimental phases, including individuals whose preference was based on less than 6 foraging steps and may thus be less representative, and obtained similar results (data available on request).
The training phase
For each bird, we analyzed the 5 training sessions as a single long session. We classified each foraging step (see definition above) into 1 of 3 possible foraging strategies: joining, searching, or secondary searching. A joining step was defined as a step in which another bird was present during, or 1 s before, the focal bird’s first peck at the well. A searching step was defined as a step in which no other bird was present during, or 1 s before, the focal bird’s first peck at the well. Secondary searching steps were searching steps to profitable variable wells (i.e., to 1 of the 5 variable wells that contained seeds) that were previously visited during the same training session; therefore, seeds in these wells were visible prior to the act of pecking. These steps were regarded as separate from the “searching steps” (above) because they provide a different experience from visiting intact profitable wells (the seeds are visible and no digging may be necessary).
For each bird, we calculated joining proportion as the proportion of joining steps in the total number of foraging steps. A successful foraging step was defined as a foraging step in which the focal bird received at least 1 seed. Accordingly, successful foraging steps include the first step in a training session to any constant well (as all of them contained 1 seed that was almost always found by the sparrows) and all steps to profitable variable wells (as profitable variable wells were never depleted during the 2min of the trial). We calculated foraging success in variable or constant wells as the proportion of successful foraging steps to each well type among the total number of steps to that type. Similarly, we calculated searching/joining success in variable or constant wells as the proportion of successful searching/joining steps to each well type among the total number of searching/joining steps to this type. Note that by our definition, foraging success is the probability of finding food, regardless of its amount. For each foraging step, we also measured the time spent at a well as an indirect measurement of food intake. The strong relationship between time spent at wells that contained seeds and actual food intake during this time was verified recently by Brenner Y and Ilan T (unpublished data) based on measurements taken under the same experimental conditions, using a high-definition camera and a viewing angle that allow an accurate count of the number of seeds eaten by 18 individuals during 5 training sessions (total of 750 seeds eaten). This analysis confirmed the strong correlation between number of seeds eaten and time spent at wells that contained seeds (N = 12, r = 0.96, P < 0.0001; N = 14, r = 0.91, P < 0.0001, for constant and variable wells, respectively) and demonstrated that seeds are eaten at a rate of 0.58 seeds/s at constant wells and 0.87 seeds/s at profitable variable wells (applying the ratio estimate procedure; Cochran 1977; the difference between these intake rates is statistically significant: t = 2.427, df = 22, P = 0.024).
Preference for variable or constant wells was calculated as the proportion of foraging steps to a well type among total number of foraging steps. For birds that showed significant preference for variable or constant wells in the test phase (see below), we investigated the effect of experience during training on well preference. To that end, we first searched for the step of preference emergence during the training phase. We did this by tracing the foraging steps of each individual from the last foraging step back, identifying the first step after which well-type preference was significantly biased in favor of either variable or constant wells (using sign test, P < 0.05). Foraging success at each well type prior to preference emergence was used to assess the effect of experience on well-type choice. We used the time spent at wells of each type after preference emergence to assess the effect of the emerged preference on individuals’ food intake.
The test phase
To minimize the potential effect of preference extinction (recall that wells contained colored sand only with no food during this phase), we analyzed only the first 20 foraging steps of the test phase. To score well-type preference, we calculated preference for green wells (variable wells during the training phase) and tested for statistical significance using a sign test (preference for yellow wells is always 1 − preference for green wells).
For statistical analysis, we used nonparametric tests. When mean values are provided, they are accompanied by ±standard error.
RESULTS
Thirty-four sparrows (17 males and 17 females) performed at least 6 foraging steps in each of the 2 experimental phases (training and test phases) and were thus included in our analysis (8, 4, 6, 2, 5, and 9 birds from the 6 flocks). For these birds, during the training phase, there were no differences between flocks in the total number of foraging steps (Kruskal–Wallis H 5 = 7.418, P = 0.191; 99.735±6.716); in joining proportion (Kruskal–Wallis H 5 = 1.472, P = 0.916; 0.116±0.022); and in the preference for variable wells (Kruskal–Wallis H 5 = 6.232, P = 0.284; 0.492±0.039). Similarly, during the test phase, there were no differences between the groups in the total number of steps (Kruskal–Wallis H 5 = 3.966, P = 0.554; 17.029±0.780) and in the preference for green wells (Kruskal–Wallis H 5 = 4.494, P = 0.481; 0.467±0.050). Accordingly, the data for sparrows from all 6 cages were pooled.
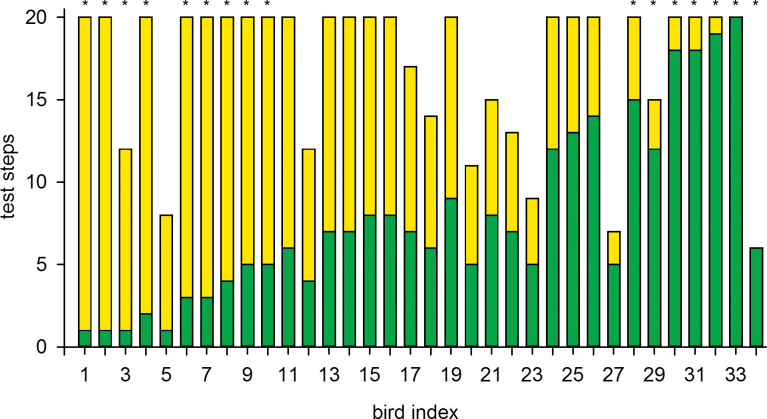
Individual well-type preference during the test phase is shown in Figure 1. Of the 34 birds, 16 (47%) showed significant well-type preference (sign test P < 0.05). Note that only 5% (2 out of 34 birds) are expected to show such a preference by chance (binomial test, P < 0.0001). Interestingly, well-type preference in the test phase was not uniform; 7 birds significantly preferred the green wells that were the variable wells during the training, and 9 birds preferred the yellow wells that were the constant wells during the training (Figure 1). The 16 birds that showed significant well-type preference originated from all 6 cages; the number of birds from each cage (preferred green wells, preferred yellow wells) was 2, 1; 0, 2; 1, 1; 1, 0; 2, 3; and 1, 2, respectively. Hence, well-type preference was not associated with any particular flock.
Figure 1.
Birds’ well-type preference during the test phase. Individuals are sorted from 1 to 34 according to their preference for green wells (number of foraging steps to green wells divided by the total number of foraging steps). For each bird, the numbers of test steps to green or yellow wells are shown in the corresponding colors. Asterisks above bars indicate significant color preference for yellow (birds 1–4 and birds 6–9) or green (birds 28–34).
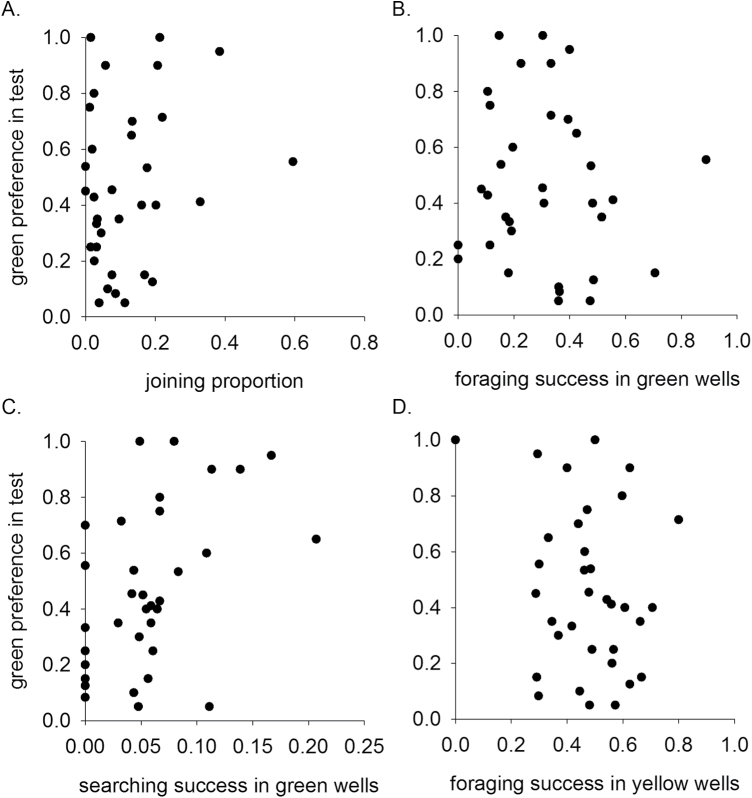
We first examined whether well-type preference may be related to social foraging strategy. During the training phase, 86% of the joining steps were to variable wells (268/312) and 95% of them (255 steps) were successful. Thus, although the probability of finding food in a variable well was initially low (0.1), once a profitable well was discovered by one individual, it created an opportunity for successful joining by its group members. These high rates of successful joining to variable wells in the training phase could have established a preference for their green color in the test phase. However, the joining proportion of individuals during the training phase was not correlated with their preference for green wells during the test phase (N = 34, r s = 0.094, P = 0.596, Figure 2A), giving no indication that success at variable green wells during joining promoted a preference for green wells during subsequent searching. In fact, even when only successful joining events in variable wells are considered, their proportion (out of all foraging steps) was not correlated with preference for green wells during the test phase (N = 34, r s = 0.118, P = 0.504).
Figure 2.
The relation between (A) joining proportion during the training phase and preference for green wells during the test phase (r s = 0.094, P = 0.596); (B) foraging success at green (variable) wells during the training phase and preference for green wells during the test phase (r s = −0.114, P = 0.521); (C) searching success at green (variable) wells and preference for green wells during the test phase (r s = 0.467, P = 0.005); and (D) foraging success at yellow (constant) wells during the training phase and preference for green wells during the test phase (r s = −0.153, P = 0.387). N = 34 in all panels.
To test whether sparrows learned to prefer the well type at which they were more successful, regardless of the social foraging strategy they used, we tested whether foraging success at variable wells during the training phase (i.e., successful visits to variable wells out of all visits to variable wells) was correlated with the preference for green wells during the test phase. No correlation was found (N = 34, r s = −0.114, P = 0.521, Figure 2B). However, when we examined only the searching steps during the training phase (excluding joining and secondary searching), searching success in variable wells (i.e., successful searching in variable wells out of all searching steps to variable wells) was significantly correlated with preference for green wells during the test phase (N = 34, r s = 0.467, P = 0.005, Figure 2C). These results suggest that well-type preference may be related to individual experience during searching, but not during joining or during secondary searching when seeds are visible and searching in the sand is unnecessary. Finally, foraging success in constant wells during the training phase (i.e., successful visits to constant wells out of all visits to constant wells) was not correlated with well-type preference during the test phase (N = 34, r s = −0.153, P = 0.387, Figure 2D).
We further analyzed the relationship between well-type preference and individual experience based on the detailed data for the 16 sparrows that developed a significant well-type preference during the test phase and for which we identified the step of preference emergence (see Methods). Three of these 16 birds could not be used for the analysis because they developed a color preference from the first step of training (i.e., their step of preference emergence was also their first training step, so previous experience was irrelevant). For the remaining 13 birds, the preference emergence step ranged from the 4th to the 144th step (48.923±12.15). An additional bird was removed from the analysis because its well-type preference in the test was different from that in the training phase (after preference emergence). A detailed examination of the foraging step history of this bird showed that it found a profitable variable well on its last training step, which may explain the shift in preference during the test. For the remaining 12 birds, well-type preference during training was in agreement with color preference in the test: 5 birds significantly preferred green, that is, variable wells, and 7 preferred yellow, that is, constant wells. These birds will be referred to as “the variable group” and “the constant group,” respectively.
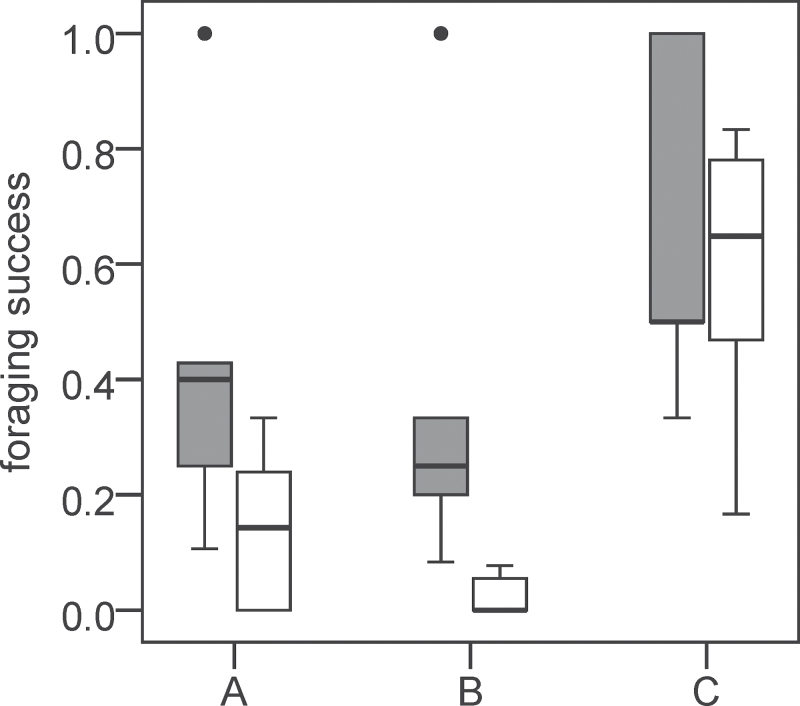
As illustrated in Figure 3, prior to preference emergence, birds of the variable group had higher success in variable wells than birds of the constant group, although this trend was not significant (Figure 3A; Mann–Whitney for variable and constant groups U = 6, P = 0.072). However, when only searching steps are considered (excluding joining and secondary searching), this difference becomes highly significant (Figure 3B; Mann–Whitney U = 0, P = 0.003). Importantly, there was no difference between the variable and the constant groups in foraging success in constant wells (Figure 3C; Mann–Whitney U = 15, P = 0.725), suggesting that individual differences in color choice were caused by the different success experienced in variable wells rather than in constant wells.
Figure 3.
Different aspects of foraging success for the variable group (gray bars, N = 5) and the constant group (white bars, N = 7) prior to preference emergence. (A) Foraging success at variable wells (Mann–Whitney U = 6, P = 0.072). (B) Searching success at variable wells (Mann–Whitney U = 0, P = 0.003). (C) Foraging success at constant wells (Mann–Whitney U = 15, P = 0.725). Bottom and top hinges in box plots represent 25th and 75th percentiles, respectively, the middle line represents the median, and whiskers represent minimum or maximum values. The 2 data points above graph A and B represent outliers from the green group (beyond 1.5 times the interquartile range).
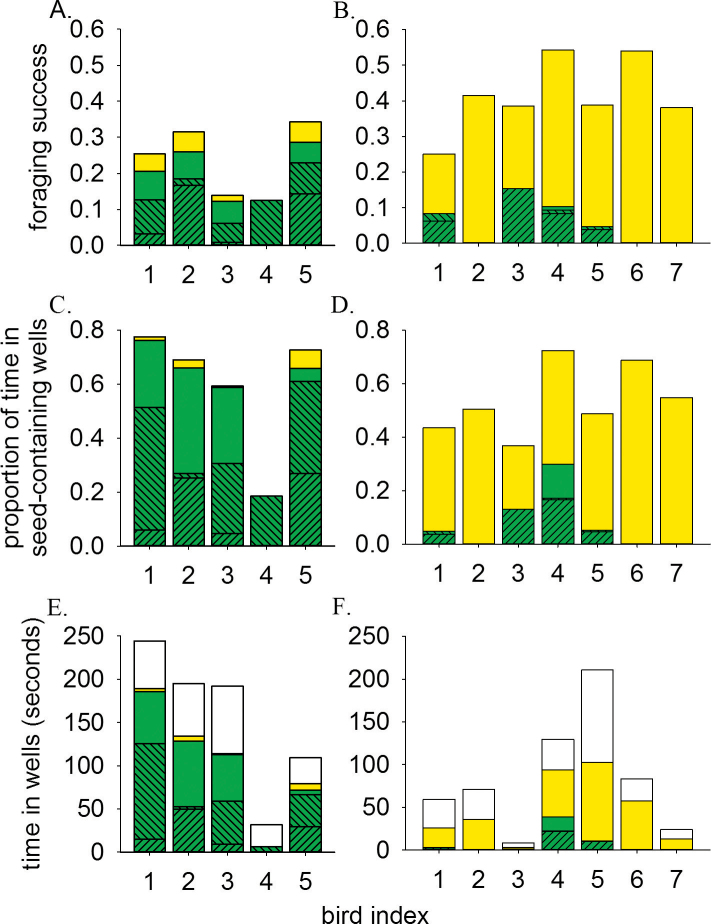
Finally, to assess whether the emerging well-type preference influenced the sparrows food intake, we analyzed foraging success (the probability of finding food) and the time spent at wells of different types (Figure 4). We did this for all training steps that followed the emergence of well-type preference. Foraging success was different between the 2 groups (Figure 4A,B; 0.235±0.045 and 0.414±0.038 for the variable and constant groups, respectively; Mann–Whitney U = 32, P = 0.018). As expected, the variable group was the one having the lower foraging success. Yet, there was no difference between the groups in the proportion of time spent at seed-containing wells (Figure 4C,D; 0.594±0.106 and 0.537±0.049 for the variable and constant groups, respectively; Mann–Whitney U = 10, P = 0.268). This is because the time spent at profitable variable wells, where many seeds are consumed, is longer than at constant wells (i.e., the lower probability of finding food in variable wells is compensated by the larger amount of reward). This result is also demonstrated by the absolute time spent in seed-containing wells, which was higher for the variable group, though not significantly so (Figure 4E,F; 104.580±30.462 and 47.343±14.702 for the variable and constant groups, respectively; Mann–Whitney U = 8, P = 0.149). Interestingly, some birds of the constant group spent considerable time foraging at variable wells, mostly as a result of joining (see Figure 4D,F). For birds from the variable group, on the other hand, most of the time spent in variable wells was as a result of either searching or secondary searching (see Figure 4C,E).
Figure 4.
Different aspects of foraging success for each bird in the variable and constant groups after the emergence of well-type preference. Top pair of charts: foraging success of the variable (A) and constant (B) group. Middle pair: the proportion of time spent at seed-containing wells for the variable (C) and constant (D) group. Bottom pair: the absolute time spent in wells for the variable (E) and constant (F) group. Striped [////] green bars: joining events to profitable variable wells; striped [\\\\] green bars: secondary searching events to profitable variable wells; plain green bars: searching events to profitable variable wells; plain yellow bars: successful visits to constant wells; plain white bars: visits to empty wells (relevant only for panels E and F and includes visits to empty variable wells or to previously visited constant wells).
DISCUSSION
Our results show that socially foraging sparrows that are trained and tested under the same conditions can nevertheless develop different risk preferences. Some of our sparrows learned to prefer the variable (high risk–high reward) wells that were covered with green sand, whereas others learned to prefer the constant (low risk–low reward) wells that were covered with yellow sand. We found that this dichotomy was not related to social foraging strategies (searching vs. joining) but to stochastic individual differences in sampling success during food searching (see also Morand-Ferron et al. 2011 for the effect of stochastic group dynamics on foraging strategy choice). Note that the relationship between well-type preference and sampling success cannot be explained by preexisting color preference. This is because stochastic differences in sampling success are clearly independent of individual color preference (i.e., whether food is found in a variable well is purely a matter of chance). For the same reason, our results could also not be explained by the birds’ previous participation in another experiment (see Methods).
In theory, if our sparrows had perfect information about the experimental setup, most of them should have preferred green wells where the expected payoff was 8 times that in yellow wells. Group competition makes the payoffs from green wells frequency dependent, so they should not be chosen in all cases. Nevertheless, the null model for optimal choice still predicts that variable wells should be preferred over constant wells at a ratio of 8:1 (following Fretwell and Lucas 1968). That our results are not consistent with this theoretical prediction is not surprising, however, given that information had to be learned from experience and that learning about rare events (i.e., success in variable wells) is inherently difficult (Hertwig et al. 2004). Indeed, sparrows that were fortunate in experiencing success in variable wells before committing themselves to constant wells were those that developed a preference for variable wells, as expected.
Considering that scroungers have more opportunities for social learning (Giraldeau 1997; Laland 2004), and following earlier theoretical analysis (Arbilly et al. 2011), we hypothesized that learning to prefer a risky patch would be easier for sparrows that scrounge more (see Introduction). However, this was not the case in our experiment (Figure 2A). Moreover, our analysis suggests that the sparrows did not use food-related cues available to them during scrounging for their subsequent foraging decisions. Instead, they seem to learn and use cues (sand color in our experiment) available during active searching. In accordance with previous studies in pigeons and zebra finches (Giraldeau and Lefebvre 1987; Beauchamp and Kacelnik 1991), the sparrows possibly ignore or give lower weight to information obtained when joining other birds or even when picking exposed seeds during secondary searching steps (Figure 3). From a mechanistic point of view, it is possible that the salience of the edible stimulus (seeds), or the image of another bird that is eating the food, captures the attention of the scrounging bird and interferes with the potential association of sand color and food (see also Beauchamp and Kacelnik 1991). However, scrounging in ravens and marmosets has been shown to facilitate social learning (Fritz and Kotrschal 1999; Caldwell and Whiten 2003), suggesting that attentional problems may not pose a real constraint on the evolution of social learning. Moreover, recent experiments in our laboratory (Truskanov N, unpublished data) show that hand-raised house sparrows can easily learn to associate sand color with food when scrounging on their artificial mother (a stuffed female sparrow on which they were imprinted). Accordingly, house sparrows are cognitively capable of social learning but may activate this ability only during an early ontogenetic stage or under specific circumstances. Social learning from a parent may be reliable as the fledgling follows the parent continuously and acquires a large sample of successes and failures for each potential cue. On the other hand, social learning during scrounging in a large flock may be less reliable because occasional foraging success of a flock member in the vicinity of a certain cue may not reveal how frequently the same cue was also associated with failures.
It is difficult to tell whether the dichotomy in well-type preference that emerged in most of our flocks was related to some aspects of group dynamics or would also emerge and persist in a population of solitary learners. One possibility is that any learner could eventually develop a preference for one well type that is difficult to reverse (e.g., March 1996). For example, the probability that an individual with a strong preference for constant wells would eventually explore and find food at a variable well may be so small that the preference for constant wells would also remain stable in a population of solitary learners. Alternatively, some aspects of group dynamics such as competition or food depletion may help to create or to maintain the observed dichotomy, so without them all birds would eventually learn to prefer the variable wells. Further work is required to explore this possibility. In particular, an experiment in which many solitary house sparrows are tested under the same conditions may reveal whether the group dynamic is necessary or not. Such an experiment is currently underway but progress is slow because adult sparrows can easily become stressed when in isolation.
The idea that a preference for low risk–low reward wells may not persist in a population of solitary sparrows is an interesting one. However, it may not be so relevant for natural populations of house sparrows, which are strongly social. It is therefore important to consider how learning mechanisms that produce such an apparently maladaptive preference in about half of the cases have not been selected against. One possibility is that our experimental setup was too extreme, so that in the real world, a preference for the constant (safe) option would not be as maladaptive as in our experiment (although there is evidence that a situation where higher expected payoff is associated with greater risk may be common in nature; Lewis 1980; Kelrick et al. 1986; Price and Reichman 1987; Edenius 1991). An alternative explanation may be suggested by our data and is based again on considering the social context and consequences of individual decisions. Although for many sparrows learning resulted in an apparently maladaptive risk aversion (preferring constant over variable), group living still allowed them to enjoy profitable green wells by occasionally scrounging on sparrows that learned to prefer the variable wells (Figure 4). Furthermore, the proportion of time spent at seed-containing wells and the absolute time spent at such wells were only slightly, but not significantly higher for the variable group. Although feeding rate in profitable variable wells was estimated to have been higher than in constant wells (0.87 vs. 0.58 seeds/s, see Methods), the payoff experienced by the birds of the variable group was certainly not 8 times higher than that experienced by the birds of the constant group. In our experimental setup, the quantitative balance of these 2 types of payoff is difficult to evaluate and may not reflect the correct balance between them under natural conditions. The argument is merely a qualitative one, but it demonstrates once again that the adaptive value of learning and decision-making mechanisms may be better understood within the broader social context under which they are likely to operate in nature.
FUNDING
This work was supported in part by the United States-Israel Bi-national Science Foundation (2004412 to A.L., U.M., and M.W.F.), by the Israel Science Foundation (1312/11 to A.L.), and by the National Institute of Health (GM028016 to M.W.F.).
Acknowledgments
We thank M. Arbilly, A. Belmaker, and 2 anonymous referees for constructive comments, A. Dekel, Y. Oren, Y. Perry, O. Yadgar, and I. Brickner for technical assistance and data collection, and the staff of the I. Meier Segals Garden for Zoological Research of Tel-Aviv University.
REFERENCES
- Arbilly M, Motro U, Feldman MW, Lotem A. 2010. Co-evolution of learning complexity and social foraging strategies. J Theor Biol. 267:573–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbilly M, Motro U, Feldman MW, Lotem A. 2011. Evolution of social learning when high expected payoffs are associated with high risk of failure. J R Soc Interface. 8:1604–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard CJ, Sibly RM. 1981. Producers and scroungers: a general model and its application to captive flocks of house sparrows. Anim Behav. 29:543–550 [Google Scholar]
- Beauchamp G. 2000. Learning rules for social foragers: implications for the producer-scrounger game and ideal free distribution theory. J Theor Biol. 207:21–35 [DOI] [PubMed] [Google Scholar]
- Beauchamp G, Kacelnik A. 1991. Effects of the knowledge of partners on learning rates in zebra finches Taeniopygia guttata. Anim Behav. 41:247–253 [Google Scholar]
- Belmaker A, Motro U, Feldman MW, Lotem A. 2012. Learning to choose among social foraging strategies in adult house sparrows (Passer domesticus). Ethology. 118:1111–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd R, Richerson PJ. 1985. Culture and the evolutionary process. Chicago (IL): University of Chicago Press [Google Scholar]
- Bro-Jørgensen J, Johnstone RA, Evans MR. 2007. Uninformative exaggeration of male sexual ornaments in barn swallows. Curr Biol. 17:850–855 [DOI] [PubMed] [Google Scholar]
- Caldwell CA, Whiten A. 2003. Scrounging facilitates social learning in common marmosets, Callithrix jacchus. Anim Behav. 65:1085–1092 [Google Scholar]
- Caraco T. 1981. Risk-sensitivity and foraging groups. Ecology. 62:527–531 [Google Scholar]
- Caraco T, Martindale S, Whittam TS. 1980. An empirical demonstration of risk-sensitive foraging preferences. Anim Behav. 28:820–830 [Google Scholar]
- Cochran WG. 1977. Sampling techniques. New York: Wiley Press [Google Scholar]
- Dall SRX, Giraldeau L-A, Olsson O, McNamara JM, Stephens DW. 2005. Information and its use by animals in evolutionary ecology. Trends Ecol Evol. 20:187–193 [DOI] [PubMed] [Google Scholar]
- Denrell J, March JG. 2001. Adaptation as information restriction: the hot stove effect. Organ Sci. 12:523–538 [Google Scholar]
- Dor R, Lotem A. 2009. Heritability of nestling begging intensity in the house sparrow (Passer domesticus). Evolution. 63:738–748 [DOI] [PubMed] [Google Scholar]
- Dubois F, Morand-Ferron J, Giraldeau L-A. 2010. Learning in a game context: strategy choice by some keeps learning from evolving in others. Proc R Soc B. 277:3609–3616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenius L. 1991. The effect of resource depletion on the feeding behaviour of a browser: winter foraging by moose on Scots pine. J Appl Ecol. 28:318–328 [Google Scholar]
- Erev I, Barron G. 2005. On adaptation, maximization, and reinforcement learning among cognitive strategies. Psychol Rev. 112:912–931 [DOI] [PubMed] [Google Scholar]
- Fawcett TW, Hamblin S, Giraldeau L-A. 2012. Exposing the behavioral gambit: the evolution of learning and decision rules. Behav Ecol. 24:2–11 [Google Scholar]
- Feldman MW, Aoki K, Kumm J. 1996. Individual versus social learning: evolutionary analysis in a fluctuating environment. Anthropol Sci. 104:209–231 [Google Scholar]
- Fretwell SD, Lucas HLJ. 1968. On territorial behavior and other factors influencing habitat distribution in birds. I. Theoretical development. Acta Biotheor. 19:16–36 [Google Scholar]
- Fritz J, Kotrschal K. 1999. Social learning in common ravens, Corvus corax . Anim Behav. 57:785–793 [DOI] [PubMed] [Google Scholar]
- Galef BG, Laland KN. 2005. Social learning in animals: empirical studies and theoretical models. BioScience. 55:489–499 [Google Scholar]
- Giraldeau L-A. 1997. The ecology of information use. In: Krebs JR, Davis NB, editors. Behavioral ecology: an evolutionary approach. Oxford: Blackwell Scientific Publications; p. 42–68 [Google Scholar]
- Giraldeau L-A, Caraco T. 2000. Social foraging theory. Princeton (NJ): Princeton University Press [Google Scholar]
- Giraldeau L-A, Dubois F. 2008. Social foraging and the study of exploitative behavior. Adv Stud Behav. 38:59–104 [Google Scholar]
- Giraldeau L-A, Lefebvre L. 1987. Scrounging prevents cultural transmission of food-finding behaviour in pigeons. Anim Behav. 35:387–394 [Google Scholar]
- Hamblin S, Giraldeau L-A. 2009. Finding the evolutionarily stable learning rule for frequency-dependent foraging. Anim Behav. 78:1343–1350 [Google Scholar]
- Hertwig R, Barron G, Weber EU, Erev I. 2004. Decisions from experience and the effect of rare events in risky choice. Psychol Sci. 15:534–539 [DOI] [PubMed] [Google Scholar]
- Ilan T. 2011. Aspects of learning in social foraging of house sparrows (Passer domesticus) [MSc thesis]. [Tel Aviv (Israel)]: Tel Aviv University; (in Hebrew with English summary). [Google Scholar]
- Kacelnik A, Bateson M. 1996. Risky theories—the effects of variance on foraging decisions. Am Zool. 36:402–434 [Google Scholar]
- Kacelnik A, Bateson M. 1997. Risk-sensitivity: crossroads for theories of decision-making. Trends Cogn Sci. 1:304–309 [DOI] [PubMed] [Google Scholar]
- Kacelnik A, Brito e Abreu F. 1998. Risky choice and Weber’s law. J Theor Biol. 194:289–298 [DOI] [PubMed] [Google Scholar]
- Kahneman D, Tversky A. 1979. Prospect theory: an analysis of decision under risk. Econometrica. 47:263–292 [Google Scholar]
- Katsnelson E, Motro U, Feldman MW, Lotem A. 2008. Early experience affects producer–scrounger foraging tendencies in the house sparrow. Anim Behav. 75:1465–1472 [Google Scholar]
- Katsnelson E, Motro U, Feldman MW, Lotem A. 2012. Evolution of learned strategy choice in a frequency-dependent game. Proc R Soc B. 279:1176–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keasar T. 2002. Bees in two-armed bandit situations: foraging choices and possible decision mechanisms. Behav Ecol. 13:757–765 [Google Scholar]
- Kelrick MI, Macmahon JA, Parmenter RR, Sisson DV. 1986. Native seed preferences of shrub-steppe rodents, birds and ants: the relationships of seed attributes and seed use. Oecologia. 68:327–337 [DOI] [PubMed] [Google Scholar]
- Laland KN. 2004. Social learning strategies. Anim Learn Behav. 32:4–14 [DOI] [PubMed] [Google Scholar]
- Lendvai AZ, Barta Z, Liker A, Bókony V. 2004. The effect of energy reserves on social foraging: hungry sparrows scrounge more. Proc R Soc B. 271:2467–2472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis A. 1980. Patch by gray squirrels and optimal foraging. Ecology. 61:1371–1379 [Google Scholar]
- March JG. 1996. Learning to be risk averse. Psychol Rev. 103:309–319 [Google Scholar]
- McNamara JM, Houston AI. 1992. Risk-sensitive foraging—a review of the theory. Bull Math Biol. 54:355–378 [Google Scholar]
- McNamara JM, Houston AI. 2009. Integrating function and mechanism. Trends Ecol Evol. 24:670–675 [DOI] [PubMed] [Google Scholar]
- Morand-Ferron J, Giraldeau L-A. 2010. Learning behaviorally stable solutions to producer-scrounger games. Behav Ecol. 21:343–348 [Google Scholar]
- Morand-Ferron J, Wu G-M, Giraldeau L-A. 2011. Persistent individual differences in tactic use in a producer-scrounger game are group dependent. Anim Behav. 82:811–816 [Google Scholar]
- Morgan D, Cézilly F, Giraldeau L-A. 2011. Personality affects zebra finch feeding success in a producer–scrounger game. Anim Behav. 82:61–67 [Google Scholar]
- Niv Y, Joel D, Meilijson I, Ruppin E. 2002. Evolution of reinforcement learning in uncertain environments: a simple explanation for complex foraging behaviors. Adapt Behav. 10:5–24 [Google Scholar]
- Price M, Reichman O. 1987. Distribution of seeds in Sonoran Desert soils: implications for heteromyid rodent foraging. Ecology. 68:1797–1811 [DOI] [PubMed] [Google Scholar]
- Real LA. 1991. Animal choice behavior and the evolution of cognitive architecture. Science. 253:980–986 [DOI] [PubMed] [Google Scholar]
- Rendell L, Boyd R, Cownden D, Enquist M, Eriksson K, Feldman MW, Fogarty L, Ghirlanda S, Lillicrap T, Laland KN. 2010. Why copy others? Insights from the social learning strategies tournament. Science. 328:208–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafir S, Reich T, Tsur E, Erev I, Lotem A. 2008. Perceptual accuracy and conflicting effects of certainty on risk-taking behaviour. Nature. 453:917–920 [DOI] [PubMed] [Google Scholar]
- Stephens DW. 1981. The logic of risk-sensitive foraging preferences. Anim Behav. 29:628–629 [Google Scholar]
- Stephens DW, Kerr B, Fernández-Juricic E. 2004. Impulsiveness without discounting: the ecological rationality hypothesis. Proc R Soc B. 271:2459–2465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd PM, Gigerenzer G. 2000. Précis of simple heuristics that make us smart. Behav Brain Sci. 23:727–741 [DOI] [PubMed] [Google Scholar]
- Trimmer PC, McNamara JM, Houston AI, Marshall JAR. 2012. Does natural selection favour the Rescorla–Wagner rule? J Theor Biol. 302:39–52 [DOI] [PubMed] [Google Scholar]
- Weber EU, Shafir S, Blais A-R. 2004. Predicting risk sensitivity in humans and lower animals: risk as variance or coefficient of variation. Psychol Rev. 111:430–445 [DOI] [PubMed] [Google Scholar]
- Wu G-M, Giraldeau L-A. 2005. Risky decisions: a test of risk sensitivity in socially foraging flocks of Lonchura punctulata. Behav Ecol. 16:8–14 [Google Scholar]