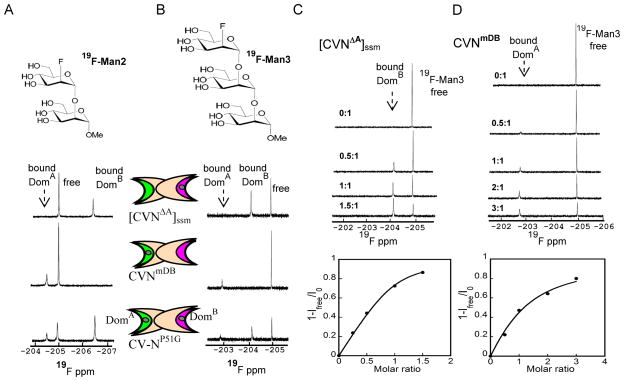
Figure 2.
1D-19F NMR spectra of 19F-Man2/Man3 in the presence of [CVNΔA]ssm, a variant that contains a single glycan binding site on domain B, CVNmDB, a variant that contains a single glycan binding site on domain A, and CV-NP51G that is wild-type-like with both glycan binding sites present, at 280 K. CV-N domains are represented by two elongated crescents, representing the two binding sites. Site 1 on domain B is colored pink and site 2 on domain A in green. A) 19F-1D NMR spectra of 200 μM 19F-Man2 in the presence of [CVNΔA]ssm, CVNmDB, and CV-NP51G. B) 19F-1D NMR spectra of 50 μM 19F-Man3 in the presence of [CVNΔA]ssm, CVNmDB and CV-NP51G. Bound-state and free-state signals are in slow exchange for [CVNΔA]ssm in C) and CVNmDB in D. In the titration curves (bottom panels) the bound fraction is derived from the signal intensity (1-Ifree/I0) during the titration, with Ifree the intensity of the free ligand signal at each point in the titration, and I0 the intensity of the free ligand signal at the beginning of the titration. In C) [CVNΔA]ssm was titrated into 50 μM 19F-Man3 (top panel), with molar ratios of protein/glycan: 0:1, 0.5:1, 1:1, 1.5:1. In D) CVNmDB was titrated into 50 μM 19F-Man3 (top panel), with protein/glycan molar ratios: 0:1, 0.5:1, 1:1, 2:1, and 3:1.