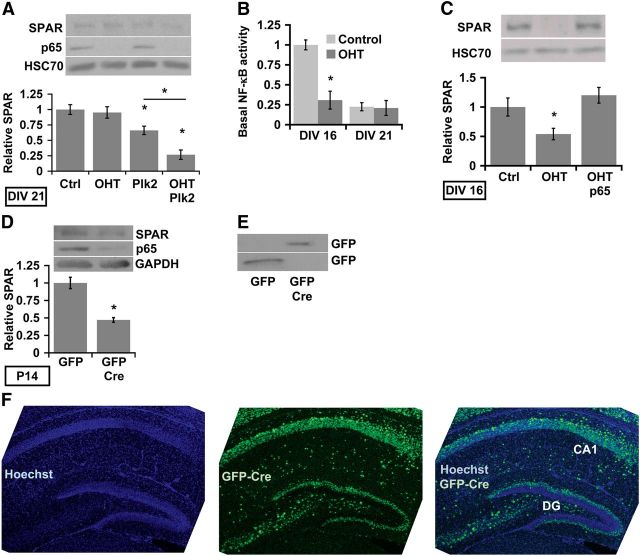
Figure 5.
NF-κB positively regulates SPAR protein in vitro and in vivo. A, NF-κB limits Plk2-induced SPAR protein loss in neurons. Representative immunoblot (inset) and quantification of SPAR levels in lysates from mock or Plk2 infected (lentivirus, 2 d) DIV21 hippocampal cultures in the presence (Ctrl) or absence of p65 (OHT). HSC70 was used as the loading control (*p ≤ 2.87 × 10−3, ANOVA; n = 5 independent experiments). B, C, Levels of neuronal SPAR positively correlate with NF-κB activity. B, NF-κB reporter assay shows that NF-κB activity is higher in developing (DIV16) compared to mature (DIV21) hippocampal cultures. C, Representative immunoblot and quantitated levels of SPAR protein in DIV16 RelAF/F hippocampal cultures in the presence (Ctrl) or absence (OHT) of p65, or following rescue of p65-deficiency by expression of exogenous p65 (OHT/p65; using lentiviral expression of Myr-GFP-F2A-p65). Average data are plotted normalized to Ctrl. HSC70 was used for the loading control (*p ≤ 0.04; n ≥ 3 independent experiments). D, Representative immunoblot (inset) and normalized quantification of SPAR protein levels in RelAF/F P14 mice transduced with control GFP or GFP-Cre by AAV injections at P0. SPAR levels are significantly reduced (p = 2.07 × 10−4; nGFP = 3, nGFP-Cre = 6 mice) by in vivo NF-κB deficiency. E, Immunoblot showing expression of GFP and GFP-Cre from RelAF/F hippocampi transduced with AAV injected at P0 and harvested at P14. F, Confocal Z-stack projections of a P12 mouse hippocampus showing viral expression of GFP-Cre following P0 lateral ventricle AAV injections. Hippocampi were cryosectioned after harvest at P12 with Hoechst dye used to counterstain nuclei. We observe >50% infection efficacy of neurons in the hippocampal pyramidal layer (CA1 shown in image) and in the dentate gyrus (DG). All error bars indicate SEM.