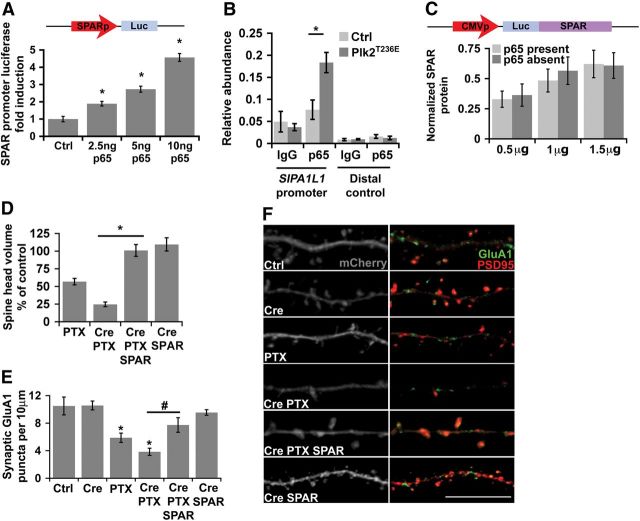
Figure 6.
SPAR is transcriptionally upregulated by NF-κB and rescues the decrease in synaptic strength due to loss of p65 in neurons exposed to hyperactivity. A, NF-κB induces a reporter driven by the SPAR promoter assayed in HEK 293T cells. Diagram (top inset) of SPAR promoter luciferase reporter, with ∼1.2 kb flanking the mouse SPAR transcriptional start site, containing four putative κB DNA binding sites. Plot of reporter assay in cells transduced with 0 to 10 ng of p65 subunit of NF-κB (*p ≤ 0.01; n = 3 independent experiments). B, NF-κB induction by Plk2 leads to an increased association of p65 with SIPA1L1 promoter in hippocampal neurons. ChIP assays were performed with either anti-p65 antibody or isotype-matched IgG, in DIV21 hippocampal dissociated cultures expressing either GFP (Ctrl) or GFP-tagged constitutively active Plk2T236E; using either primers within the SIPA1L1 promoter (left) or upstream control primers (right; *p = 0.008; n = 5 independent experiments). The relative abundance of specific amplicons in the IP compared to the input DNA is shown on the y-axis. C, NF-κB does not regulate SPAR protein levels from a construct lacking the endogenous SPAR promoter in neurons. Inset, Diagram of construct containing the SPAR coding sequence fused directly to luciferase to allow sensitive quantification, and driven by a constitutive CMV promoter. SPAR protein level is quantified by luciferase activity from RelAF/F DIV16 dissociated hippocampal cultures transfected with a dose titration of the fusion construct in the presence or absence of p65. D, Expression of exogenous SPAR rescues the overshoot of spine head volume loss in p65-deficient (Cre) neurons exposed to elevated activity. Average spine head volumes after chronic PTX are plotted as a percentage of control neurons (100%) not exposed to PTX to illustrate that low levels of SPAR expression rescue the exaggerated loss of spine volume in p65-deficient neurons exposed to elevated activity (*p = 1.87 × 10−8, ANOVA; Cre/PTX/SPAR; n ≥ 10 dendritic segments from 4 independent experiments), but do not alter average spine volumes without heightened activity (Cre/SPAR). E, Exogenous SPAR expression opposes excessive loss of surface synaptic GluA1 in response to elevated activity (PTX) in p65-deficient (Cre) neurons. Plot of average surface synaptic GluA1 density for the indicated conditions. Synaptic GluA1 was quantified (Imaris, Bitplane) from confocal images as surface GluA1 puncta colocalized with transfected PSD95 puncta in DIV21 RelAF/F hippocampal pyramidal neurons (*p ≤ 3.39 × 10−3, #p = 2.16 × 10−3, ANOVA; n ≥ 10 dendritic segments from 4 independent experiments). F, Representative confocal projections of dendrites from pyramidal neurons for the indicated conditions, as quantified in D and E. Right panels are masked (Imaris) for ubiquitously expressed mCherry fluorophore (left) to allow confirmation by visual inspection that illustrated puncta are within the presented dendritic segments. Scale bar, 10 μm. All error bars indicate SEM.