Abstract
It is still unclear whether microvascular complications of type 2 diabetes correlate with leukocyte-endothelium interactions and/or myeloperoxidase (MPO) levels. In the present study, we found that serum levels of glucose, the rate of ROS and MPO concentration were higher in type 2 diabetic patients. Patients with nephropathy (39.6%) presented higher MPO levels that correlate positively with the albumin/creatinine ratio (r=0.59, p<0.05). In addition, nephropatic patients showed increased leukocyte-endothelium interactions due to an undermining of polymorphonuclear leukocytes (PMN) rolling velocity and increased rolling flux and adhesion, which was accompanied by a rise in levels of the proinflammatory cytokine tumour necrosis factor alpha (TNFα) and the adhesion molecule E-selectin. Furthermore, MPO levels were positively correlated with PMN rolling flux (r=0.855, p<0.01) and adhesion (r=0.682, p<0.05). Our results lead to the hypothesis that type 2 diabetes induces oxidative stress and an increase in MPO levels and leukocyte-endothelium interactions, and that these effects correlate with the development of nephropathy. Antioxid. Redox Signal. 19, 1452–1458.
Introduction
Hyperglycemia frequently induces endothelial dysfunction in diabetic patients, which can lead to micro- and macro-vascular complications (5). The severity of type 2 diabetic complications normally correlates with plasma glucose levels, and the inflammation provoked by their deregulation can contribute to insulin resistance, not only in the adipose tissue, but also in target organs, such as liver, kidney or skeletal muscle, thereby aggravating the diabetic state.
Low-grade chronic inflammation and endothelial dysfunction contribute to the initiation and progression of microvascular complications associated with type 2 diabetes. In fact, endothelial dysfunction parallels microalbuminuria in diabetes, while diabetes leads to ultrastructural alterations in the glomerular filtration barrier and loss of endothelial factors carriers in the kidney (2). Diabetic nephropathy contributes in a large way to the main causes of mortality related with diabetes, such as cardiovascular disease (CVD), cardiac insufficiency and infectious processes. The presence of microalbuminuria in diabetic patients can be associated with higher mortality, and, if arterial hypertension is also present, the risk of mortality is even greater.
Innovation.
To date, there are few studies specifically focusing on the relationship between myeloperoxidase (MPO) levels and type 2 diabetes and their results are controversial. Our results show that the concentration of MPO in type 2 diabetes patients is notably elevated and there is a positive correlation between MPO levels and diabetic nephropathy. Furthermore, levels of cytokines, adhesion molecules, and leukocyte-endothelium interactions are increased in type 2 diabetes patients and these increases are exacerbated by the presence of nephropathy. These observations raise the question of a putative role of MPO in the development of diabetic nephropathy mediated by endothelial dysfunction.
It has been suggested that myeloperoxidase (MPO), a heme protein derived from leukocytes, plays a key role in leukocyte-mediated vascular injury responses in inflammatory CVD. MPO is released from activated leukocytes at inflammatory sites, generating reactive oxygen species (ROS). However, the antimicrobial activity of MPO can also produce oxidative damage in the endothelium and vessel wall; thus, promoting CVD and clinical complications (9). Only a few studies have explored the relationship between MPO and type 2 diabetes, and their results are controversial.
The inflammatory response to a bacterial infection is initiated when leukocytes migrate from the blood into the affected tissue. This results in adhesion of leukocytes to the vascular endothelium and chemotax towards the site of infection, where bacteria are killed by phagocytosis and ROS generation. Bacteria clearance is impaired in type 2 diabetes, which affects blood circulation; thus, increasing the recruitment of leukocytes (8). Diabetes-related complications are often related with the endothelial dysfunction caused by hyperglycemia, which usually involves increased leukocyte-endothelial interactions and, therefore, leukocyte recruitment. However, contradictory data exist regarding leukocyte-endothelial cell interactions in models of inflammation, with both higher and lower rates having been reported, as well as elevated levels of adhesion molecules.
The present study was carried out to evaluate the relationship between MPO, leukocyte activation and the increase of leukocyte-endothelium interactions observed in type 2 diabetes and to explore a possible correlation between these factors and nephropathy.
Clinical and Metabolic Characteristics
The anthropometric and metabolic characteristics of type 2 diabetic patients and control subjects are presented in Table 1. Fasting levels of serum glucose were higher in type 2 diabetic patients (p<0.05) (Table 1). Chronic complication of type 2 diabetes in the form of nephropathy was detected among some (39.6%) of our subjects. In terms of the oral antidiabetic treatment received by patients, 58.2% were treated with metformin, 8.95% with glitazone, and 39.7% with sulphonylurea or glinide. About 39.6% of patients received insulin treatment. With respect to hypolipemiant treatment, 46.1% of patients received statins and 8.0% received fibrate. With regard to antihypertensive drugs, 42.4% of patients received angiotensin-converting enzyme inhibitor or angiotensin II-receptor treatment and 25.0% received diuretic treatment.
Table 1.
Subject Baseline Characteristics
| Controls | Type 2 diabetic patients | |
|---|---|---|
| Age (years) | 50.1±7.6 | 53.4±6.7 |
| Duration of diabetes mellitus (years) | 13.1±9.2 | |
| Hypertension | 40.9% | 52.8% |
| Mean SBP (mmHg) | 132.4±4.9 | 137.6±2.3 |
| Mean DBP (mmHg) | 82.5±11.9 | 77.1±9.4 |
| Body mass index (kg/m2) | 28.8±0.9 | 30.2±0.4 |
| Active smoker | 18.0% | 19.0% |
| Blood glucose (mg/dl) | 102.6±23.3 | 165.9±64.9a |
| HbA1c (%) | — | 7.2±1.6 |
Variables are expressed as mean±standard deviation. The results of categorical variables are expressed in percentages.
p<0.05 respect to control.
DBP, diastolic blood pressure; HbA1c, glycosylated haemoglobin; SBP, systolic blood pressure.
ROS production and MPO levels
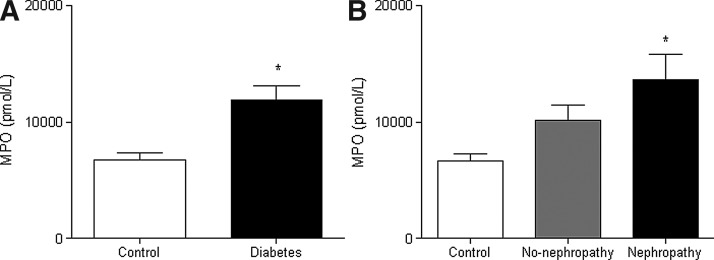
DCFH-DA fluorescence was significantly higher in leukocytes from type 2 diabetic patients (Fig. 1A), as seen also by static cytometry (Fig. 1B) and fluorescence microscopy (Fig. 1C); thus, showing an increase in ROS production and, consequently, conditions of oxidative stress. Therefore, we evaluated the levels of MPO in serum from type 2 diabetic patients and controls and found that they were higher among the former (p<0.05, Fig. 2A). In addition, we assessed MPO levels of patients grouped according to the presence or absence of nephropathy. MPO levels were significantly higher in patients with nephropathy compared to controls, but not in patients without nephropathy compared to controls (Fig. 2B). A correlation study revealed a positive correlation between serum MPO levels and the urinary albumin: creatinine ratio in type 2 diabetic patients with nephropathy (r=0.59, p<0.05).
FIG. 1.
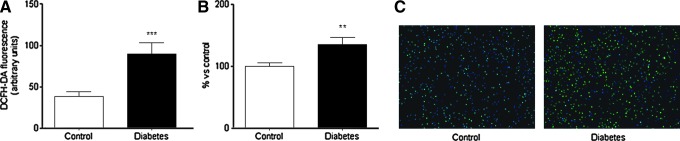
Effects of type 2 diabetes on levels of ROS in PMN. (A) Alterations in the fluorescence of DCFH-DA measured by fluorimetry; (B) Mean DCFH fluorescence assessed by static cytometry;% versus control. (C) DCFH-DA fluorescence in PMNs assessed by fluorescence microscopy; nuclei: Hoechst 33342 signal (blue); ROS: DCFH-DA signal (green). **p<0.01 ***p<0.001 versus control. DCFH-DA, 2′,7′-dichlorodihydrofluorescein diacetate; PMN, polymorphonuclear leukocytes; ROS, reactive oxygen species. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
FIG. 2.
Effects of type 2 diabetes on MPO concentration. Serum MPO concentration compared between type 2 diabetic patients and control subjects (A) and between type 2 diabetic patients with or without nephropathy and control subjects (B). *p<0.05. MPO, myeloperoxidase.
Adhesion assay under flow conditions
In light of the correlation observed between MPO levels and nephropathy in our type 2 diabetic patients, and due that pathophysiological states are characterized by leukocyte recruitment to the arterial wall, we evaluated the leukocyte-endothelium interactions in the three groups of subjects (controls and type 2 diabetic patients with and without nephropathy, according to the albumin/creatinin ratio). Type 2 diabetes was related to a decrease in the rolling velocity of polymorphonuclear leukocytes (PMN) that was more evident among patients with nephropathy (Fig. 3A; p<0.01 in patients without nephropathy and p<0.001 in those with nephropathy with respect to controls, and p<0.05 when non-nephropathic patients were compared with their nephropathic counterparts). A significant increase was observed in rolling flux (cells per minute), (Fig. 3B; p<0.05 in patients without nephropathy and p<0.001 in those with nephropathy with respect to the control group, and p<0.001 comparing non-nephropathic vs. nephropathic patients) and PMN adhesion (cells per square millimeter) (Fig. 3C; p<0.05 in patients without nephropathy and p<0.01 in those with nephropathy with respect to the control group, and p<0.05 when comparing non-nephropathic and nephropathic patients). These results suggest that type 2 diabetes is related with an increase in leukocyte-endothelium interactions that is more pronounced among patients with nephropathy than those who do not develop this complication (Fig. 3A–C).
FIG. 3.
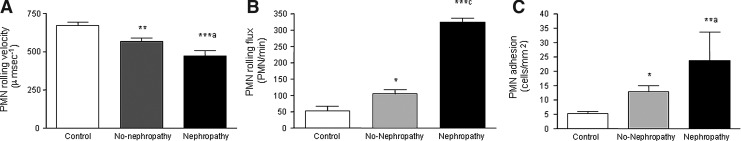
Effects of type 2 diabetes on leukocyte-endothelium interactions. Effects of type 2 diabetes with and without nephropathy on (A) PMN rolling velocity (microsecond-1), (B) rolling flux (PMN per minute), and (C) PMN adhesion (PMN per square millimetre). *p<0.05, **p<0.01 and ***p<0.001 versus Control. ap<0.05 and cp<0.001 corresponds with the differences between type 2 diabetic patients with nephropathy versus those without nephropathy.
In addition, we explored a possible correlation between leukocyte-endothelial interactions and MPO levels in all our subjects and found that MPO levels were positively correlated with PMN rolling flux (r=0.855, p<0.01) and adhesion (r=0.682, p<0.05) (Table 2).
Table 2.
Correlation Coefficient Between Leukocyte-Endothelial Interactions and MPO Levels
| |
MPO (pM) |
|
|---|---|---|
| r | p-Value | |
| Rolling velocity | −0.394 | 0.263 |
| Rolling flux | 0.855 | 0.004 |
| Adhesion | 0.682 | 0.030 |
Correlation coefficients were estimated by Spearman's correlation. We have studied the possible correlation between leukocyte-endothelial interactions and MPO levels in all subjects. We found that MPO levels were positively correlated with PMN rolling flux (r=0.855, p<0.01) and adhesion (r=0.682, p<0.05).
MPO, myeloperoxidase; PMN, polymorphonuclear leukocytes.
Levels of cytokines and adhesion molecules
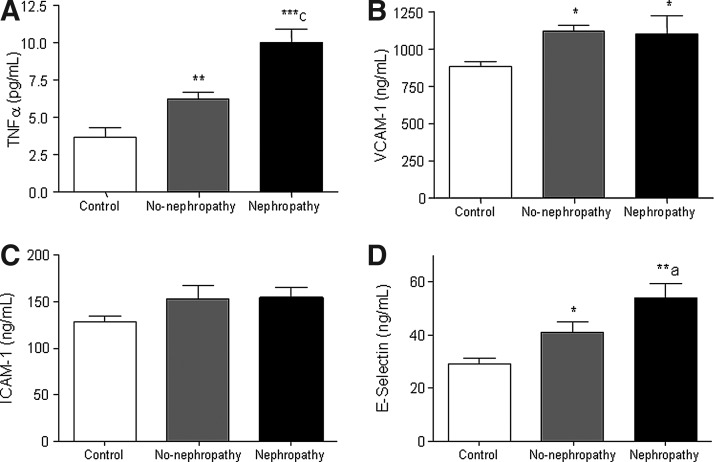
Type 2 diabetes produced a significant increase in the levels of TNFα that was more evident in patients with nephropathy (Fig. 4A; p<0.01 without nephropathy and p<0.001 with nephropathy with respect to control subjects, and p<0.001 when comparing non-nephropathic versus nephropathic patients). Type 2 diabetic patients presented an increase in the adhesion molecules vascular cell adhesion molecule-1 (VCAM-1) (Fig. 4B; p<0.05 in both groups) and E-selectin (Fig. 4D; p<0.05 without nephropathy and p<0.01 with nephropathy with respect to controls and p<0.05 when comparing non-nephropathic versus nephropathic patients). Increased levels of the proinflammatory cytokine TNFα and adhesion molecules were related with type 2 diabetes, and the increase in these levels was higher among patients with nephropathy than those without nephropathy, with the exception of VCAM-1 (p<0.05).
FIG. 4.
Cytokines and adhesion molecules in the serum of type 2 diabetic patients with nephropathy and control subjects. (A) TNFα, (B) VCAM-1, (C) ICAM-1, and (D) E-selectin. Data are expressed as mean±SD. *p<0.05, **p<0.01, ***p<0.001 with respect to control group; ap<0.05, cp<0.001 when comparing non-nephropathic versus nephropathic patients using one way ANOVA with post hoc Student-Newman-Keuls test. ICAM-1, intercellular adhesion molecule-1; TNFα, tumour necrosis factor alpha; VCAM-1, vascular cell adhesion molecule-1.
Type 2 diabetes is associated with oxidative stress and microvascular dysfunction. Although endothelial dysfunction and endothelial/leukocyte interactions are key features of diabetes and are thought to be a major cause of diabetes-associated vascular complications, the underlying molecular mechanisms remain unclear.
It has been demonstrated that ROS play a key role in hyperglycemia-mediated endothelial dysfunction and microvascular complications in type 2 diabetes (1). Our results confirm that type 2 diabetes is related to an increase in ROS production in PMN, as demonstrated in a previous study (3). Levels of MPO were higher in the leukocytes of type 2 diabetic patients, and these increases seemed to be due to the presence of nephropathy. In these subgroups of patients, a positive correlation between levels of serum MPO and urinary albumin:creatinine ratio was apparent. MPO seems to play an important role in endothelial dysfunction. The activated leukocyte-derived hydrogen peroxide (H2O2), produced by the nicotinamide adenine dinucleotide phosphate-oxidase (NADPH oxidase) is acutely released in respiratory bursts and disappears quickly. In contrast, activated leukocyte-released MPO binds to the vascular wall for a considerable time, sometimes for several days releasing ROS continuously.
Few studies have focused specifically on the relation between MPO levels and the presence of type 2 diabetes. Some clinical studies have reported significantly higher MPO levels in diabetics, whereas others have found no correlation, and lower levels in some cases (6). Such discrepancies may be explained; at least, in part, by differences in methodology, tissues analyzed, and study populations that hamper the interpretation of data. Regarding the possible mechanisms linking MPO and type 2 diabetes, it has been speculated that high MPO activity in diabetes is related to hyperinsulinemia, as the number of adhered neutrophils (evaluated based on MPO activity in vitro) rose when patients were administered insulin. It has also been proposed that vascular-bound MPO uses high glucose-stimulated H2O2 to induce glucose-induced injury to the vascular wall. The findings in our study are in accordance with reports demonstrating higher levels of MPO in diabetic patients.
Pathophysiological states, such as atherosclerosis and hypertension are characterized by leukocyte recruitment to the arterial wall. To study this process, we have used an in vitro model in which human leukocytes flow over a monolayer of human endothelial cells with a shear stress similar to that observed in vivo (7). This reproduces the process that precedes inflammation in vivo (rolling and adhesion) and which is critical to homeostasis and vascular cell integrity. If these interactions are exacerbated, the vascular dysfunction and injury associated with many CVDs can occur.
Type 2 diabetes can induce leukocyte-endothelium interactions and an increase in proinflammatory cytokines and adhesion molecules (4). We have observed that the inflammatory state in type 2 diabetic patients undermines PMN rolling velocity and enhances rolling flux and adhesion, thereby inducing leukocyte-endothelium interactions, and that there is a simultaneous increase in the proinflammatory cytokine TNFα and the adhesion molecules VCAM-1 and E-selectin. In type 2 diabetic patients with nephropathy, levels of proinflammatory cytokine TNFα, E-selectin and the induction of leukocyte-endothelium interactions are exacerbated. In this context, the function and number of endothelial progenitor cells, the maintenance of vascular homeostasis, and endothelium repair have all been proposed as pathogenic mechanisms of vascular disease in diabetes, thereby highlighting the important link between endothelial function and diabetic nephropathy.
Furthermore, we found a positive correlation between MPO levels and leukocyte-endothelium interactions, especially at the levels of rolling flux and adhesion. These results suggest that the aggravated inflammatory state underlying nephropathy could lead to an increase in the production of MPO by leukocytes that would enhance leukocyte-endothelium interactions, as the MPO molecule is thought to be capable of attracting leukocytes to the vascular wall via its electrostatic characteristics.
In conclusion, we demonstrate an increase in the rate of ROS in diabetic patients and elevated concentrations of MPO in type 2 diabetes patients in general and, particularly, in those with nephropathy. In addition, MPO levels correlate positively with the albumin:creatinine ratio. Inflammation in type 2 diabetes induces leukocyte-endothelium interactions with a simultaneous increase in TNFα and the adhesion molecules VCAM-1 and E-selectin, and these conditions are aggravated by the presence of diabetic nephropathy.
Given that ROS and MPO are enhanced in type 2 diabetic patients and that MPO levels are specifically correlated with diabetic nephropathy, MPO/ROS may represent an important pathway underlying microvascular complications in type 2 diabetes, such as nephropathy.
Notes
Materials and Methods
Subjects
Our study population consisted of 203 patients (age 53.4±6.7) consecutively diagnosed with type 2 diabetes at the Endocrinology Outpatient's Department of the University Hospital Dr. Peset (Valencia, Spain) according to criteria of the American Diabetes Association. Diagnosis was confirmed when a patient fulfilled one or more of the following criteria: (i) levels of fasting serum glucose ≥7.0 mM (126 mg/dl) or random serum glucose ≥11.1 mM (200 mg/dl) on at least two occasions; (ii) HbA1c ≥6.5%; or (iii) antidiabetic medication. Patients taking allopurinol or any oral antioxidant supplements were excluded from the study.
Control subjects (50.1±7.6) were enrolled in the study after confirming that they did not fulfill any of the following criteria: (i) levels of fasting serum glucose ≥7.0 mM (126 mg/dl) or random serum glucose ≥11.1 mM (200 mg/dl) on at least two occasions; (ii) HbA1c ≥6.5%; or (iii) antidiabetic medication. An additional inclusion criterion was the absence of any documented history of vascular disease (ischemic cardiopathy, peripheral arteriopathy, or cerebrovascular accident) and diabetes. Exclusion criteria were physical disability or alterations in the electrocardiogram, or clinical signs of coronary artery, peripheral, or cerebrovascular disease. None of the controls had nephropathy.
In accordance with the Declaration of Helsinki, all participants were informed of the purpose, procedures, risks, and possible benefits of the study and gave their express consent to take part. Approval was obtained from the hospital's ethics committee.
During a patient's first visit, his/her medical history was taken (including current treatment) and a physical examination was conducted to record his/her weight, height, and body mass index.
Evaluation of nephropathy
Diabetic nephropathy was confirmed when the urinary albumin:creatinine ratio was ≥30 mg/g. Albumin was determined in a 24-h urine sample by means of immunoturbidimetry.
Biochemical parameters
Patients were given an appointment to attend the Endocrinology Service Functional Test Unit a week later (between 8:00–10:00 am) to have blood taken for clinical chemistry tests, and were given instructions to fast for at least 10 h beforehand. Blood samples were stored in SST tubes and immediately centrifuged for 10 min at 2000 g at 4°C. Serum aliquots were obtained on the same day as extraction to measure glucose and HbA1c. The intraserial coefficient of variation for this test was <3.5.
Cells
PMNs were isolated from citrated blood samples and incubated with dextran (3%) for 45 min. The supernatant was obtained from the Fycoll-Hypaque gradient and centrifuged at 250 g for 25 min. The resulting pellet was then centrifuged at 100 g for 5 min at room temperature, and then resuspended in lysis buffer. PMNs were counted, washed in HBSS medium, and resuspended in complete RPMI medium.
Human umbilical vein endothelial cell culture
Human umbilical vein endothelial cells (HUVEC) were harvested from umbilical cords by means of treatment with collagenase (9). Passage 1 from primary cultures was employed in subsequent experiments. For adhesion studies, HUVEC were cultured on fibronectin (5 mg/ml)-coated 25-mm plastic coverslips until confluent (∼48 h).
Measurement of ROS production
Total ROS production was evaluated in PMNs by two methods. Cells were incubated (30 min) with the fluorescent probe (5×10−6 M) 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA). First, ROS production was assessed by fluorimetry using a Synergy Mx plate reader (BioTek Instruments) and second, it was assessed using a fluorescence microscope (IX81; Olympus) coupled with the static cytometry software “ScanR” version 2.03.2 (Olympus). For static cytometry, PMNs from each subject were seeded in 48-well plates in triplicate and 16 images per well were recorded and analyzed.
MPO assay
Serum MPO concentrations were measured using an immunoassay based on a double-antibody “sandwich” technique according to the manufacturer's instructions (MPO EIA kit; Cayman Chemical). In brief, this assay provides a fluorescence-based method for detecting the MPO peroxidase activity in crude cell lysates. The assay utilizes the peroxidase component of MPO, and the reaction between H2O2 and 10-acetyl-3,7-dihydroxyphenoxazine (ADHP) produces the fluorescent compound resorufin.
Adhesion assay. Levels of cytokines and adhesion molecules
The parallel plate flow chamber in vitro model has been described in detail elsewhere (7). To perform adhesion assays, coverslips containing confluent HUVEC monolayers were inserted into a circular recess in the bottom plate of the flow chamber (maintained at 37°C), to which a 5×25 mm portion of the endothelial cells were exposed. The chamber was mounted on an inverted microscope (Nikon Eclipse TE 2000-S) connected to a video camera. Experiments were performed using a 40×objective lens. PMNs were resuspended in Dulbecco's Phosphate-Buffered Saline containing 20×10−3 M HEPES and 0.1% human serum albumin at 1×106 cells/ml, and were drawn across the HUVEC monolayer at a controlled flow rate of 0.36 ml/min (estimated shear stress of 0.7 dyne/cm2). A circular glass window in the top plate of the chamber allowed real time microscopic examination of the monolayer exposed to the flow. Images in a single field of view were recorded over a 5-min period, which allowed leukocyte parameters to be determined. Leukocyte rolling was calculated by counting the number of leukocytes rolling over 100 μm2 of the endothelial monolayer during a 1-min period. The velocity of 20 consecutive leukocytes in the field of focus was determined by measuring the time required to travel a distance of 100 μm.
Adhesion was evaluated by counting the number of PMN that maintained stable contact with the HUVEC for 30 s. Tumoral necrosis factor (TNFα, 10 ng/ml, 4 h) and platelet activating factor (1 μM, 1 h) were used as positive controls for HUVEC and leukocytes, respectively. A Luminex 100 flow analyzer system was employed to analyze VCAM-1, E-selectin, intercellular adhesion molecule-1 (ICAM-1), and TNFα in serum from controls and type 2 diabetic patients (Millipore).
Data analysis
Quantitative variables are expressed as mean and standard deviation if normally distributed and median and quartiles when otherwise. All statistical analyses were performed using Graph Pad Software. A Student's t test for unpaired samples was used to compare the means of normally distributed variables, while the Mann-Whitney U test was employed for variables without a normal distribution. A one-way ANOVA and post hoc Student-Newman-Keuls test were performed for multiple comparisons. Significance was defined as *p<0.05, **p<0.01, and ***p<0.001. A correlation test was performed using the Pearson correlation coefficient (r).
Abbreviations Used
- CVD
cardiovascular disease
- DBP
diastolic blood pressure
- DCFH-DA
2′,7′-dichlorodihydrofluorescein diacetate
- H2O2
hydrogen peroxide
- HbA1c
glycosylated haemoglobin
- HUVEC
human umbilical vein endothelial cells
- ICAM-1
intercellular adhesion molecule-1
- MPO
myeloperoxidase
- NADPH oxidase
nicotinamide adenine dinucleotide phosphate-oxidase
- PMN
polymorphonuclear leukocytes
- ROS
reactive oxygen species
- SBP
systolic blood pressure
- TNFα
tumour necrosis factor alpha
- VCAM-1
vascular cell adhesion molecule-1
Acknowledgments
We thank B. Normanly for his editorial assistance (University of Valencia) and Isabel Soria for her work in the extraction of biological samples (University Hospital Dr. Peset).
S.R., M.R., and R.F. researched data. V.M.V., C.P., A.A., A.J., and A.H. reviewed/edited the manuscript. A.H., M.R., and A.A. contributed to the discussion and reviewed/edited the manuscript. S.R. researched the data and contributed to the discussion. V.M.V and A.H. wrote the manuscript. A.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
This study was financed by grants PI10/1195, PI12/1984, SAF2010-16030, CIBERehd CB06/04/0071, PROMETEO 2010/060, ACOMP/2013/061, ACOMP/2012/042, and ACOMP/2012/045. V.M.V. and M.R. are recipients of contracts from the Ministry of Health of the Valencian Regional Government and Carlos III Health Institute (CES10/030 and CP10/0360, respectively). C.d.P. was funded by Ministerio de Ciencia e Innovación [FPI grant BES-2008-004338] and by Fundación Juan Esplugues. A.A. was supported by Ministerio de Ciencia e Innovación [Ramón y Cajal program RYC2005-002295 and I3 program]. S.R. is recipient of a predoctoral fellowship from Carlos III Health Institute (FI11/00637).
References
- 1.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 2.Haraldsson B. Nystrom J. Deen WM. Properties of the glomerular barrier and mechanisms of proteinuria. Physiol Rev. 2008;88:451–487. doi: 10.1152/physrev.00055.2006. [DOI] [PubMed] [Google Scholar]
- 3.Hernandez-Mijares A. Rocha M. Apostolova N. Borras C. Jover A. Bañuls C. Sola E. Victor VM. Mitochondrial complex I impairment in leukocytes from type 2 diabetic patients. Free Radic Biol Med. 2011;50:1215–1221. doi: 10.1016/j.freeradbiomed.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 4.Hernandez-Mijares A. Rocha M. Rovira-Llopis S. Bañuls C. Bellod L. de Pablo C. Alvarez A. Roldan-Rorres I. Sola-Izquierdo E. Victor VM. Human leukocyte/endothelial cell interactions and mitochondrial dysfunction in type 2 diabetic patients and their association with silent myocardial ischemia. Diabetes Care. 2013 doi: 10.2337/dc12-1224. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hink U. Li H. Mollnau H. Oelze M. Matheis E. Hartmann M. Skatchkov M. Thaiss F. Stahl RA. Warnholtz A. Meinertz T. Griendling K. Harrison DG. Forstermann U. Munzel T. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res. 2001;88:E14–E22. doi: 10.1161/01.res.88.2.e14. [DOI] [PubMed] [Google Scholar]
- 6.Sato N. Kashima K. Tanaka Y. Shimizu H. Mori M. Effect of granulocyte-colony stimulating factor on generation of oxygen-derived free radicals and myeloperoxidase activity in neutrophils from poorly controlled NIDDM patients. Diabetes. 1997;46:133–137. doi: 10.2337/diab.46.1.133. [DOI] [PubMed] [Google Scholar]
- 7.Victor VM. Rocha M. Bañuls C. Alvarez A. de Pablo C. Sanchez-Serrano M. Gomez M. Hernandez-Mijares A. Induction of oxidative stress and human leukocyte/endothelial cell interactions in polycystic ovary syndrome patients with insulin resistance. J Clin Endocrinol Metab. 2011;96:3115–3122. doi: 10.1210/jc.2011-0651. [DOI] [PubMed] [Google Scholar]
- 8.Vozarova B. Weyer C. Lindsay RS. Pratley RE. Bogardus C. Tataranni PA. High white blood cell count is associated with a worsening of insulin sensitivity and predicts the development of type 2 diabetes. Diabetes. 2002;51:455–461. doi: 10.2337/diabetes.51.2.455. [DOI] [PubMed] [Google Scholar]
- 9.Zhang C. Yang J. Jennings LK. Leukocyte-derived myeloperoxidase amplifies high-glucose—induced endothelial dysfunction through interaction with high-glucose—stimulated, vascular non—leukocyte-derived reactive oxygen species. Diabetes. 2004;53:2950–2959. doi: 10.2337/diabetes.53.11.2950. [DOI] [PubMed] [Google Scholar]