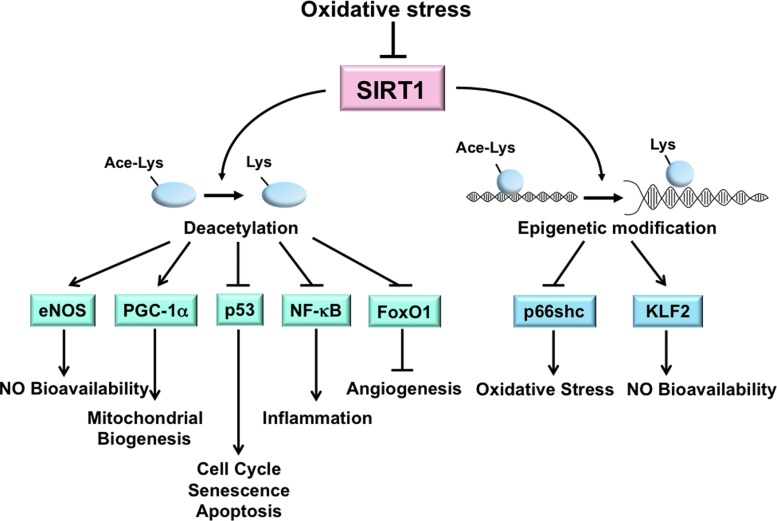
FIG. 1.
Oxidative stress-reduced SIRT1 expression or activity contributes to dysfunctional endothelium. SIRT1 directly deacetylates multiple targets, including eNOS, PGC-1α, p53, NF-κB, and FoxO1, and regulates the transcription of proteins such as p66shc and KLF2 via epigenetic modifications (i.e., histone deacetylation) in ECs. In sum, the functional outcome is enhanced NO bioavailability, mitochondrial biogenesis, and angiogenesis, but decreased senescence, apoptosis, inflammation, and oxidative stress. The increased oxidative stress associated with various pathophysiological conditions impairs SIRT1 deacetylation of these targets resulting in a dysfunctional endothelium. SIRT1, sirtuin 1; eNOS, endothelial nitric oxide synthase; PGC-1α, peroxisome proliferator-activated receptor-γ coactivator 1-alpha; p53, tumor protein 53; NF-κB, nuclear factor-kappaB; FoxO1, Forkhead box O family 1; KLF2, Krüppel-like factor 2; ECs, endothelial cells. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars