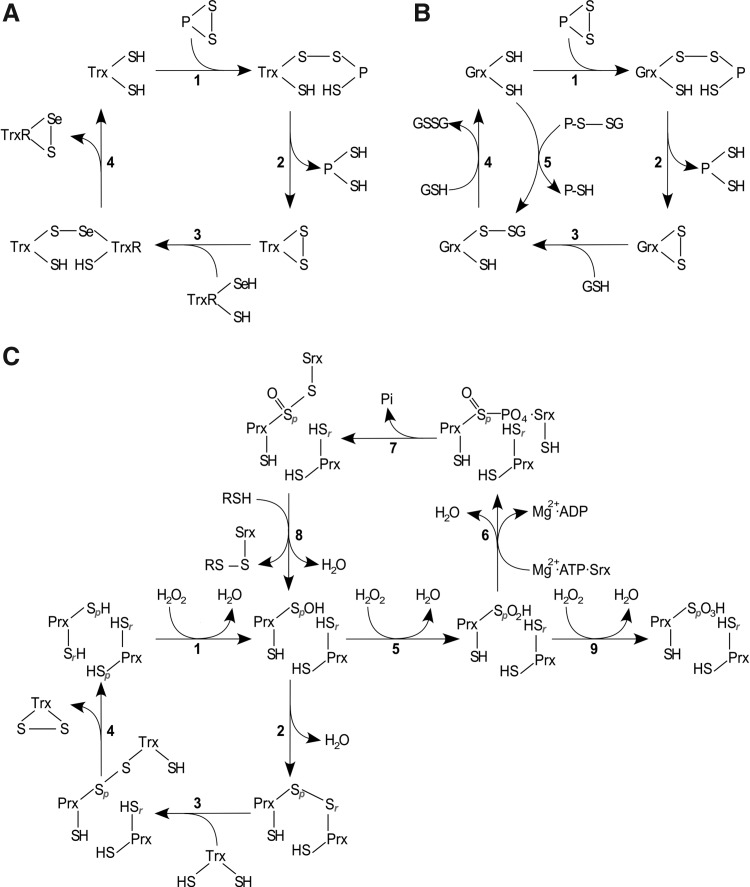
FIG. 4.
Reaction mechanisms of Trx family proteins. (A) Trxs reduce protein disulfides via the dithiol mechanism, depending on both active site cysteines. The N-terminal active site Cys forms a covalently bound mixed disulfide intermediate (A 1), which is reduced by the C-terminal active site Cys, releasing the reduced protein (A 2). Oxidized Trx is reduced by TrxR in a similar reaction sequence (A 3–4). (B) Grxs also reduce protein disulfides via the dithiol mechanism, being reduced by two GSH molecules (B 1–4). In addition, they reduce glutathionylated proteins via the monothiol mechanism (B 5–4), only depending on the N-terminal active site Cys, that attacks the GSH moiety and forms a GSH-mixed disulfide intermediate (B 5), which is reduced by another GSH molecule (B 4). (C) During the reduction of H2O2 by Prxs, the redox-active, peroxidatic Cys (labeled p) is oxidized to sulfenic acid (C 1), which either forms an inter-(2-Cys Prxs) (C 2) or an intramolecular disulfide (atypical 2-Cys Prxs) (not shown) with the resolving Cys residue (labeled r), with both being reduced by Trx as outlined in (A) (C 3–4). 1-Cys Prxs lack an additional resolving cysteine and are reduced by GSH (not shown). In the presence of H2O2, the sulfenic acid can be further oxidized (“over-oxidized”) to sulfinic acid [5] and sulfonic acid [9]. Sulfinic acid-modified Prxs can be recovered by the ATP-dependent action of sulfiredoxin (Srx) [6–8]. For a detailed discussion, see section I.A.1. H2O2, hydrogen peroxide.