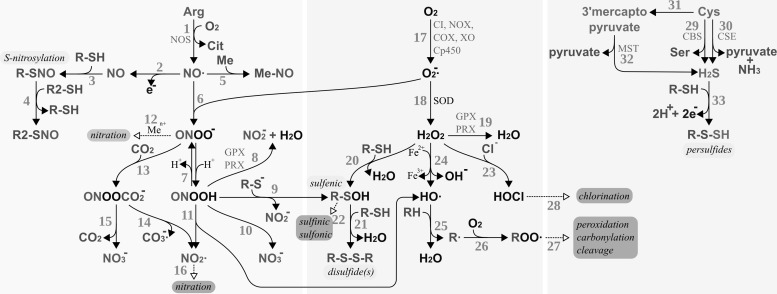
FIG. 6.
Production and reactivity of reactive nitrogen, oxygen, and sulfur species. RNS (bio-)chemistry, left side: [1] Production of nitric oxide by nitric oxide synthase (NOS). [2–3] S-nitrosylation of protein thiols. [4] Trans-nitrosylation between protein thiols. [5] Reaction of nitric oxide with metals, for example, heme iron. [6] Nitric oxide reacts spontaneously with superoxide yielding peroxynitrite. [7] Reversible protonation of peroxynitrite to peroxynitrous acid. [8] Reduction of peroxynitrous acid by glutathione peroxidases (GPxs) or PRX. [9] Peroxynitrous acid reacts with protein thiolates, yielding protein sulfenic acids. [10] Spontaneous decomposition of peroxynitrous acid yielding nitrite anion. [11] Spontaneous decomposition of peroxynitrous acid to hydroxy radicals and NO2·. [12] Peroxynitrite can (metal catalyzed) lead to the nitration of, for instance, protein tyrosyl residues. [13] Peroxynitrite and carbon dioxide react spontaneously to nitrosoperoxycarbonate. [14] Spontaneous decay of nitrosoperoxycarbonate to carbonate radical anions and nitrite radicals. [15] Spontaneous decay of nitrosoperoxycarbonate to carbon dioxide and nitrate. [16] Nitration may also be initiated by NO2·. ROS (bio-)chemistry, bmiddle: [17] Production of superoxide by, for instance, mitochondrial complex I (CI), NADH oxidase (NOX), cyclooxygenases (COX), xanthine oxidase (XO), or cytochrome P450 enzymes (Cp450). [18] Superoxide is either reduced to H2O2 or oxidized to molecular oxygen (not shown) by superoxide dismutases (SOD). [19] H2O2 can be reduced to water by GPxs or PRX. [20] H2O2 may react directly with specific thiols, yielding sulfenic acids. [21] Sulfenic acids can react with other thiols, yielding disulfides. These disulfides are direct substrates of Trxs and Grxs (not depicted). [22] Sulfenic acids may be further irreversibly oxidized, for example, by H2O2, to sulfinic and sulfonic acids. [23] H2O2 may react with chloride anions, yielding hypochlorous acid. [24] The metal-catalyzed Fenton reaction yields hydroxyl anions and hydroxy radicals. [25] Hydroxy radicals remove hydrogen from volatile organic compounds, yielding water and alkyl radicals. [26–27] Alkyl radicals may react with molecular oxygen and other compounds, eventually resulting in the peroxidation, carbonylation, or cleavage of the organic molecules, for example, proteins. [28] Hypochloric acid may lead to the chlorination of organic compounds. RSS biochemistry, right side: [29–32] Hydrogen sulfide may be the product of cystathionine β-synthase [29, CBS], cystathionine γ-lyase [30, CSE], or via 3-mercaptopyruvate sulfurtransferase [31–32, MST]. [33] Hydrogen sulfide may react with thiols in the presence of an electron and hydrogen acceptor to persulfides. Modifications labeled with a light gray background are reversible and important in redox signaling, and modifications with a dark gray background are irreversible modifications; hence, “oxidative damage.” ROS, reactive oxygen species; RNS, reactive nitrogen species; RSS, reactive sulfur species.