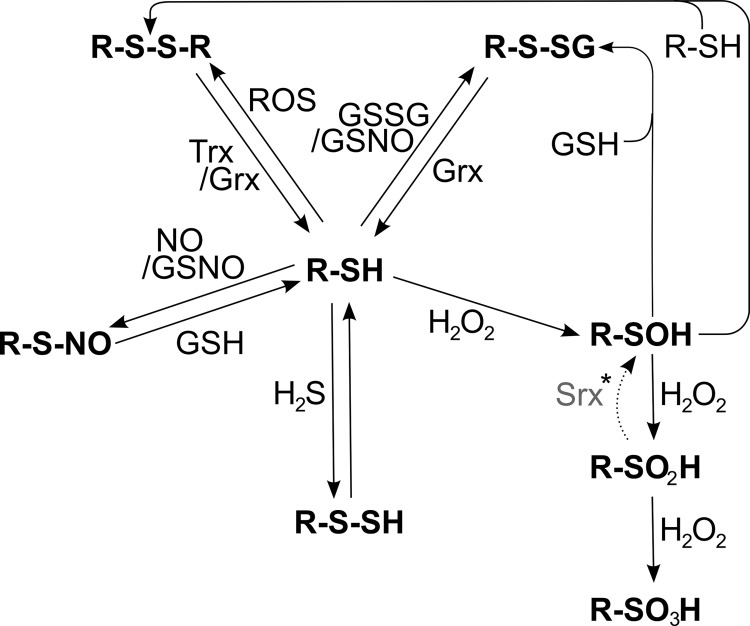
FIG. 7.
Redox modifications at cysteinyl residues. Free thiol groups (R-SH) can be reversibly modified by ROS, leading to the formation of protein disulfides (R-S-S-R), which can be reduced by the Trx and Grx systems. Thiols can also be glutathionylated (R-S-SG) by oxidized glutathione (GSSG) or S-nitroso glutathione (GSNO). The de-glutathionylation is exclusively catalyzed by Grxs. GSNO or ·NO, in general, can lead to the nitrosylation of cysteinyl residues, which can be reversed by GSH or transferred to other thiols such as the active site of Trx1 (53) (trans-nitrosylation, not shown). Another modification, induced by peroxides, is the formation of sulfenic acid (R-SOH). In the presence of another free thiol, it can be modified to a protein disulfide. However, in the presence of excessive peroxides, it can be irreversibly over-oxidized to sulfinic (R-SO2H) and sulfonic acid (R-SO3H). *The reduction of sulfinic acids to sulfenic acids, catalyzed by Srxs, is specific for Prxs; in addition, Srxs have been reported to catalyze the de-glutathionylation of Prxs.