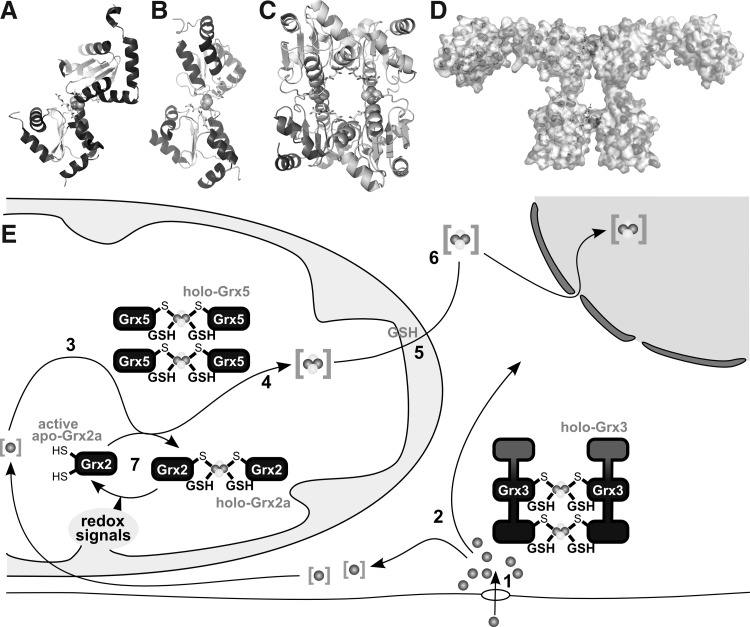
FIG. 9.
[FeS]-Grxs in cellular iron metabolism. (A) Structure of the holo-Grx2 complex consisting of two monomers Grx2 (cartoon graphics), two GSH molecules (ball and stick model), and the [2Fe2S] cluster (calotte model), derived from PDB entry 2HT9 (348). (B, C) Structures of the holo-Grx5 complex depicted as dimer (B) and tetrameric holo complexes (C), derived from PDB entry 2WUL (350). (D) Hypothetical model of the dimeric Grx3 holo complex (271). (E) Iron taken up into the cell, simplified in [1], is shuttled through the cytosol, presumably involving Grx3 [2]. Inside mitochondria, iron is used, for instance, for the biogenesis of iron-sulfur clusters [3] on a scaffold protein and transferred to target apo-proteins [4] in a reaction that requires Grx5. The export of iron-sulfur clusters in a hitherto unknown form requires GSH [5]. This compound X is used by the cytosolic iron-sulfur cluster assembly machinery for the synthesis of cytosolic and nuclear FeS proteins [6]. [7] Grx2 is usually present in the enzymatically inactive FeS-bridged dimeric holo form. On redox signals, the FeS cluster dissociates, yielding active monomeric Grx2.