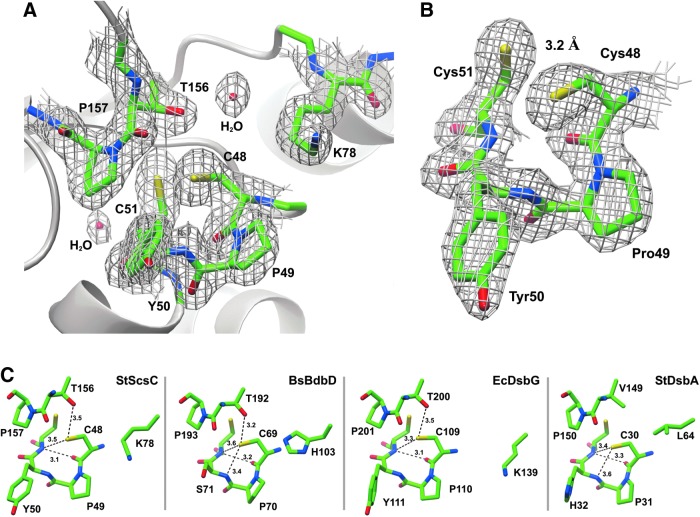
FIG. 4.
Active site regions of StScsC and structural homologues. (A) Active sites of StScsC showing the catalytic Cys-Pro-Tyr-Cys motif, the adjacent cisPro loop (Thr156-cisPro157) and the sidechain of Lys78, which protrudes into the solvent to form a positively charged lip over the active site. The 2Fo-Fc map is also shown contoured at 1.3 σ. (B) Close-up view of the StScsC active site, refined in the reduced form (2Fo-Fc maps contoured at 1.3 σ). (C) Active site regions of StScsC, BsBdbD, EcDsbG, and StDsbA showing the hydrogen bond interactions stabilizing the side chain of the nucleophilic cysteine in each active site. Hydrogen bonds are depicted by dashed lines.