Abstract
The Abl2/Arg nonreceptor tyrosine kinase is enriched in dendritic spines where it is essential for maintaining dendrite and synapse stability in the postnatal mouse brain. Arg is activated downstream of integrin α3β1 receptors and it regulates the neuronal actin cytoskeleton both by directly binding F-actin and via phosphorylation of substrates including p190RhoGAP and cortactin. Neurons in mice lacking Arg or integrin α3β1 develop normally through postnatal day 21 (P21), however by P42 mice exhibit major reductions in dendrite arbor size and complexity, and lose dendritic spines and synapses. As a result, mice with loss of Arg and Arg-dependent signaling pathways have impairments in memory tasks, heightened sensitivity to cocaine, and vulnerability to corticosteroid-induced neuronal remodeling. Therefore, understanding the molecular mechanisms of Arg regulation may lead to therapeutic approaches to treat human psychiatric and neurodegenerative diseases in which neuronal structure is destabilized.
Keywords: Abl2/Arg, integrin α3β1, actin cytoskeleton, dendritic spine, neuronal stability, cocaine, corticosteroid
Introduction
Neurons in the developing brain dynamically reorganize their dendritic branches and spines in order to acquire elaborate morphologies and integrate into active signaling networks. Once formed, however, neuronal structure must be maintained for extended periods of time to ensure proper connectivity. In fact, the destabilization of neuronal structure is commonly observed in many psychiatric and neurodegenerative diseases, where it is a contributing factor that compromises brain function. Research over the past decade has elucidated distinct signaling cascades that regulate long-term dendrite and dendritic spine stability (Lin and Koleske, 2010). This review will focus on Arg, a central regulator of neuronal stability. Arg (Abl-related gene, or Abl2) and its paralog Abl are the vertebrate members of the Abl nonreceptor tyrosine kinase family that also includes C. elegans Abl, and Drosophila (D−) Abl. These kinases play fundamental roles in translating extracellular signals from adhesion and growth factor receptors into cytoskeletal rearrangements that power changes in cell shape and movement (Bradley and Koleske, 2009).
Arg is most highly expressed in the brain, where it is enriched in dendritic spines, reaching a concentration of 400–600nM (Koleske et al. , 1998). The Arg N-terminal half has tandem SH3, SH2, and kinase domains. The SH3-SH2 domains form an inhibitory scaffold that engages the back of the kinase domain and holds it in an inactivate state (Nagar et al. , 2003). Engagement of the SH3, SH2, or kinase domains with cell-surface receptors, kinase substrates, and adaptor proteins is believed to release this inhibitory conformation and allow kinase activation. Additionally, following this release, kinase activation can be reinforced by phosphorylation of tyrosine residues in the activation loop between the SH2 and kinase domain (Bradley and Koleske, 2009, Tanis et al. , 2003). Abl family kinases also contain unique C-terminal extensions that interact with cytoskeletal regulators and directly with the actin and microtubule cytoskeletons. In particular, Arg, has at least three tandem SH3 domain binding motifs (P-XX-P), two distinct F-actin binding domains, and a microtubule binding domain (Bradley and Koleske, 2009, MacGrath and Koleske, 2012, Miller et al. , 2004, Wang et al. , 2001).
Functions
Arg signaling is essential for a variety of diverse physiological roles beyond the scope of this review, including breast cancer invasion and metastasis, viral, bacterial, and parasite infection, T cell development, and neurotransmitter release (Bradley and Koleske, 2009). This review will focus on Arg function in the regulation of neuronal structural stability.
Dendrites and synapses develop normally in the hippocampus and cortex of mice lacking arg and are indistinguishable from wild type littermates in size and morphology by weaning at postnatal day 21 (P21). However, these structures are destabilized by early adulthood (P42), leading to significantly smaller dendrite arbors and a 30% loss of synapses, Figure 1 (Gourley et al. , 2012, Moresco et al. , 2005, Sfakianos et al. , 2007). Knockdown of arg (argKD) in neuronal cultures recapitulates the dendrite morphology and dendritic spine reductions found in vivo, indicating that Arg functions cell-autonomously to control morphological stability (Lin et al. , 2013). In mice, the loss of dendrites and synapses correlates temporally with the onset of impairment in novel object recognition, a behavior that is dependent on proper hippocampal and cortical connectivity. For example, arg−/− mice can identify an object as novel at P21, but lose this ability as they age to adulthood.
Figure 1. Phenotype of arg−/− hippocampal CA1 neurons.
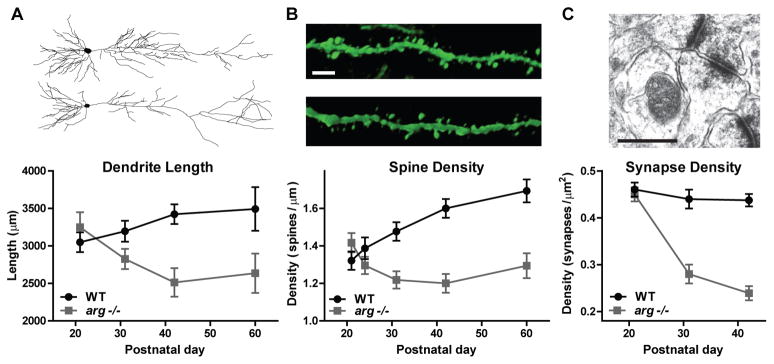
A, Representative reconstructions obtained from dye-filled live neurons. Dendrites develop normally in arg−/− mice through postnatal day 21 (P21). However, dendrites begin to regress around P31 and dendrite length (here) and complexity (not shown) are significantly reduced by P42. B, Representative confocal micrographs from apical dendrites labeled by thy1-GFP transgene expression. Scale bar 10 μm. Spines densities arg−/− neurons in are comparable to WT early in development, but by P31 are significantly reduced. C, Representative electron micrograph of a Schaffer-collateral synapse. Scale bar 500nm. Synapses density is normal at P21, but reduced by 30% in arg−/− mice at P31 and P42. Data represented in graphs obtained from (Gourley et al., 2012, Moresco et al., 2005, Sfakianos et al., 2007) and representative images modified from (Kerrisk et al., 2013, Warren et al., 2012).
Arg has also been implicated in structural changes produced in response to cocaine administration. Chronic exposure to drugs of abuse causes dendritic spine rearrangements, behavioral inflexibility, drug sensitization, and ultimately addiction. For example, repeated cocaine administration reduces dendritic spine density and increases the head size of spines that remain within the prefrontal cortex (Gourley et al., 2012). The dendrites in arg−/− mice already contain fewer spines and the remaining spines do not enlarge in response to cocaine. As a result, Arg-deficient mice have an increased psychomotor response and behavioral sensitivity to cocaine administration (Gourley et al. , 2009, Gourley et al., 2012). Alterations in other key components of the Arg signaling cascade may also contribute to cocaine-induced pathology. For example, integrin β1 receptor, a major Arg regulator in the brain, shows increased expression following cocaine exposure (Wiggins et al. , 2009) and its loss results in exaggerated psychomotor sensitivity to cocaine (Warren et al. , 2012).
The stress hormone corticosterone contributes to structural remodeling of dendrites and dendritic spines in multiple brain regions including the hippocampus, prefrontal cortex, and amygdala. Repeated stressor exposure induces immediate structural rearrangements (Vyas et al. , 2002), as well as persistent loss of dendritic arbors and spines in distinct brain regions (Gourley et al. , 2013). Interestingly, mice with a reduced gene dosage of the Arg substrate p190RhoGAP are vulnerable to behavioral and structural impairments at sub-threshold corticosterone exposure (Gourley et al., 2013), suggesting that Arg-mediated signaling events may contribute to neuronal response to stressor exposure. Future studies should identify the specific biochemical mechanism(s) responsible for this vulnerability.
Cascades
p190RhoGAP-RhoA control of dendrite stability
The Rho GTPase inhibitor p190RhoGAP (p190) is a major substrate of Arg. In neurons, active p190 inhibits RhoA GTPase to regulate dendrite arbor size (Hernandez et al. , 2004, Sfakianos et al., 2007). Arg phosphorylates p190 on Y1105, which, along with a second phosphorylation site Y1087, promotes p190 binding to two SH2 domains in p120RasGAP (p120). p120 uses its PH and CaLB domains to recruit the p190:p120 complex to the plasma membrane of cells where it attenuates RhoA GTPase activity at the cell edge (Bradley et al. , 2006). Elevated RhoA activity in neurons destabilizes dendrites via downstream effectors including ROCKII (Sfakianos et al., 2007, Threadgill et al. , 1997). Thus, Arg signaling through p190:p120 complex in neurons acts as a brake on RhoA activation to preserve dendrite stability. Reducing RhoA or ROCKII signaling in arg−/− mice and argKD cultures blocks hippocampal dendrite loss. However, this reduction in RhoA activity does not rescue synapse loss, resulting in fully elaborated dendritic arbors that have significantly fewer spines (Lin et al., 2013, Sfakianos et al., 2007). These data suggest Arg acts via additional mechanisms to regulate dendritic spine and synapse stability.
NMDA receptor control of dendritic spine and synapse stability
Our lab has recently found that the reduction of dendritic spine density in argKD cultures can be rescued by blocking NMDA receptor activity via inhibition of the NR2B subunit with ifenprodil (Lin et al., 2013). This treatment does not rescue dendrite destabilization resulting in neurons with diminished dendrite arborization, but normal dendritic spine densities along the remaining branches. Furthermore, argKD neurons have altered synaptic transmission with a lower frequency and higher amplitude of mEPSCs consistent with the reduced number of synapses and larger spine head size (Lin et al., 2013). NMDA receptor expression and function is regulated by tyrosine phosphorylation of the intracellular tail of NR2B (Gladding and Raymond, 2011), however, mechanistic details of an interaction between Arg and the NMDA receptor are unknown. Future experiments should elucidate whether and how Arg interacts with the NR2B subunit to control synapse and spine stability.
Cortactin control of dendritic spine and synapse stability
Actin is highly enriched in dendritic spines and its proper regulation is essential for spine morphology and dynamics (Fischer et al. , 1998). Cortactin is an actin regulatory protein that promotes the nucleation and stabilization of F-actin via NTA domain interactions with the Arp3-subunit of the Arp2/3 complex (Weaver et al. , 2001). Cortactin was identified as a substrate of Arg in an unbiased proteomic screen (Boyle and Koleske, 2007) and subsequently these two proteins were shown to interact through a series of binding and phosphorylation events. The cortactin SH3 domain binds to a specific P-XX-P motif in Arg, and integrin-mediated adhesion stimulates Arg to phosphorylate cortactin. This phosphorylation creates a second binding site in cortactin for the SH2 domain of Arg (Lapetina et al. , 2009). Arg binding to actin filaments can also stimulate cortactin recruitment, allowing cortactin to bind to the actin filament at a higher density (MacGrath and Koleske, 2012). These Arg:cortactin interactions are a critical trigger of cell-edge protrusions in non-neuronal cells following simulation by growth factors or adhesion to extracellular matrix (ECM) (Lapetina et al., 2009, Mader et al. , 2011).
In neurons, cortactin localizes to dendritic spines and knockdown of Arg in cultured neurons reduces the amount of cortactin and F-actin in spine heads by 40% (Lin et al., 2013). Fusion of the Arg C-terminal domain to cortactin lacking its SH3 domain mimics an “activated” Arg-bound cortactin. This fusion protein relocalizes to dendritic spine heads and prevents both the loss of F-actin and reduction of dendritic spine density in argKD neurons, but does not protect from dendrite loss (Lin et al., 2013). These results support a model in which Arg regulates dendrites and dendritic spines via independent mechanisms. In mature neurons, spine and arbor stability are uncoupled allowing the neuron to simultaneously maintain overall structural stability, while remaining plastic in response to changes in activity. Dendrite stabilization is regulated by an Arg-p190-RhoA signaling axis, while Arg interactions with the NMDA receptor and cortactin control the morphology and dynamics of dendritic spines, Figure 2.
Figure 2. Model of Arg kinase signaling pathways in hippocampal dendritic spines.
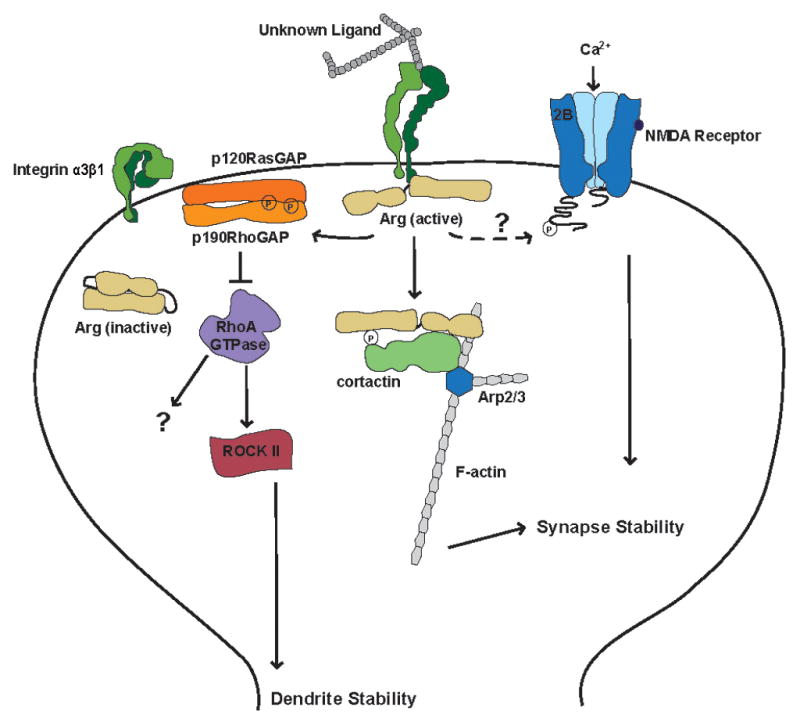
Integrin α3β1 is activated by an unknown upstream extracellular ligand. Integrin activation promotes binding of the intracellular tail of integrin β1 to Arg and activation of Arg kinase activity. Arg phosphorylates downstream substrates including p190RhoGAP (p190) and cortactin. Phosphorylated p190 allows complex formation with p120RasGAP (p120) at the post-synaptic membrane, which inhibits RhoA GTPase activity and promotes dendrite stability. Arg also phosphorylates and binds to cortactin, which promotes the nucleation and stabilization of F-actin by interactions with the Arp2/3 complex. Arg-cortactin interactions promote the stability of dendritic spines. Inhibition of the NR2B subunit of the NMDA receptor can block dendritic spine reductions associated with loss of Arg suggesting that Arg may interact with this receptor, but the mechanism of this interaction is unknown.
Key molecules
Integrins
Integrins are a class of adhesion receptors that serve as physical and functional links between the ECM and cytoskeletal control pathways (Campbell and Humphries, 2011). There are 18 known integrin heterodimers of αβ-subunits and a subset of these are expressed in neurons where they are enriched at synaptic membranes (Kerrisk et al. , 2013, Pinkstaff et al. , 1999). Integrin receptors in the brain function have diverse roles in regulating neuronal migration, synaptic plasticity, and synapse and dendrite morphology (Wu and Reddy, 2012). Arg binds directly and specifically to the intracellular tail of integrin β1 (Warren et al., 2012). Recently integrin α3 has been identified as the major partner for β1 that regulates Arg-mediated dendrite and dendritic spine maintenance (Kerrisk et al., 2013).
Possible extracellular ligands
The neuronal ECM includes a vast number of molecules that activate cell-surface receptors such as integrin α3β1. ECM components are secreted from both neurons and glial cells in the brain to influence neuronal development, structure, maintenance, plasticity, and regeneration. Laminins are a major class of integrin α3β1 ligands and they regulate neuronal migration and stability in vitro (Dityatev et al. , 2010). For example, neurons cultured on laminin-coated plates respond by increasing the number and length of neurite branches. However, neurons cultured from arg−/− mice show no response to laminin, suggesting laminin may influence neuronal morphology via Arg (Moresco et al., 2005). Additional candidate ligands include Reelin (Dulabon et al. , 2000), Netrins (Stanco et al. , 2009), and Semaphorin7A (Moresco et al., 2005), each of which can bind integrin α3β1 and activate downstream signaling. Future studies should identify which of these potential ligands engage integrin α3β1 to regulate Arg kinase signaling and thereby control dendrite maintenance and synapse stability.
Associated pathologies and therapeutic implications
Reductions in dendrite arbor size and complexity, dendritic spine and synapse densities, and synaptic connectivity are common pathologies in many psychiatric and neurodegenerative disorders (Lin and Koleske, 2010). Mutations and deletions of genes encoding Arg signaling partners, including integrin α3, integrin β1, Arg, p190, and Rho-family GTPases, are found in human patients with impaired intellectual ability (Kerrisk et al., 2013, Lin and Koleske, 2010, Lin et al., 2013). Furthermore, single-nucleotide polymorphisms in laminin genes potentially upstream of Arg signaling have been identified in cocaine addiction (Mash et al. , 2007), autism spectrum disorders (O’Roak et al. , 2011), intellectual disability (Matejas et al. , 2010), and schizophrenia (van Schijndel et al. , 2009). Continued investigation of these signaling molecules and their biochemical interactions should reveal mechanistic details of their disruption in psychiatric and neurodegenerative diseases and if they can be targeted therapeutically to stabilize neuronal structure and ameliorate disease.
Signaling Network Facts.
Abl2/Arg is unique among nonreceptor tyrosine kinases because of its ability to directly bind the actin cytoskeleton and microtubules.
Neurons in mice that lack Arg develop normally, but by late adolescence arg−/− mice have significantly smaller dendrite arbors, reduced numbers of dendritic spines and synapses, altered synapse morphology, and impairments in behavior.
Arg differentially regulates dendrite maintenance and synapse stability via distinct biochemical mechanisms.
Mutations in Arg signaling networks have been implicated in a variety of human disorders, including autism spectrum disorders, drug addiction, and schizophrenia.
Acknowledgments
We thank A.D. Levy and M.H. Omar for careful reading and editing of this manuscript.
Abbreviations
- Arg
Abl-related gene
- F-actin
filamentous actin
- SH2,SH3
Src Homology Domain 1,2
- P21,P42
postnatal day 21, 42
- argKD
arg knockdown
- p190
p190RhoGAP, 190 kD GTPase activating protein for Rho
- p120
p120RasGAP, 120 kD GTPase activating protein for Ras
- PH
pleckstrin homology domain
- CaLB
Ca2+-dependent phospholipid binding domain
- NMDA
N-methyl-D-aspartate receptor
- mEPSC
miniature evoked postsynaptic current
- NTA
N-terminal acidic domain
- ECM
extracellular matrix
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Boyle SN, Koleske AJ. Use of a chemical genetic technique to identify myosin IIb as a substrate of the Abl-related gene (Arg) tyrosine kinase. Biochemistry. 2007;46:11614–20. doi: 10.1021/bi701119s. [DOI] [PubMed] [Google Scholar]
- Bradley WD, Hernandez SE, Settleman J, Koleske AJ. Integrin signaling through Arg activates p190RhoGAP by promoting its binding to p120RasGAP and recruitment to the membrane. Molecular biology of the cell. 2006;17:4827–36. doi: 10.1091/mbc.E06-02-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley WD, Koleske AJ. Regulation of cell migration and morphogenesis by Abl-family kinases: emerging mechanisms and physiological contexts. Journal of cell science. 2009;122:3441–54. doi: 10.1242/jcs.039859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell ID, Humphries MJ. Integrin structure, activation, and interactions. Cold Spring Harbor perspectives in biology. 2011:3. doi: 10.1101/cshperspect.a004994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dityatev A, Schachner M, Sonderegger P. The dual role of the extracellular matrix in synaptic plasticity and homeostasis. Nature reviews Neuroscience. 2010;11:735–46. doi: 10.1038/nrn2898. [DOI] [PubMed] [Google Scholar]
- Dulabon L, Olson EC, Taglienti MG, Eisenhuth S, McGrath B, Walsh CA, et al. Reelin binds alpha3beta1 integrin and inhibits neuronal migration. Neuron. 2000;27:33–44. doi: 10.1016/s0896-6273(00)00007-6. [DOI] [PubMed] [Google Scholar]
- Fischer M, Kaech S, Knutti D, Matus A. Rapid actin-based plasticity in dendritic spines. Neuron. 1998;20:847–54. doi: 10.1016/s0896-6273(00)80467-5. [DOI] [PubMed] [Google Scholar]
- Gladding CM, Raymond LA. Mechanisms underlying NMDA receptor synaptic/extrasynaptic distribution and function. Molecular and cellular neurosciences. 2011;48:308–20. doi: 10.1016/j.mcn.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Gourley SL, Koleske AJ, Taylor JR. Loss of dendrite stabilization by the Abl-related gene (Arg) kinase regulates behavioral flexibility and sensitivity to cocaine. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:16859–64. doi: 10.1073/pnas.0902286106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Olevska A, Warren MS, Taylor JR, Koleske AJ. Arg kinase regulates prefrontal dendritic spine refinement and cocaine-induced plasticity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:2314–23. doi: 10.1523/JNEUROSCI.2730-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Swanson AM, Koleske AJ. Corticosteroid-induced neural remodeling predicts behavioral vulnerability and resilience. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:3107–12. doi: 10.1523/JNEUROSCI.2138-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez SE, Settleman J, Koleske AJ. Adhesion-dependent regulation of p190RhoGAP in the developing brain by the Abl-related gene tyrosine kinase. Current biology : CB. 2004;14:691–6. doi: 10.1016/j.cub.2004.03.062. [DOI] [PubMed] [Google Scholar]
- Kerrisk ME, Greer CA, Koleske AJ. Integrin alpha3 Is Required for Late Postnatal Stability of Dendrite Arbors, Dendritic Spines and Synapses, and Mouse Behavior. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:6742–52. doi: 10.1523/JNEUROSCI.0528-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koleske AJ, Gifford AM, Scott ML, Nee M, Bronson RT, Miczek KA, et al. Essential roles for the Abl and Arg tyrosine kinases in neurulation. Neuron. 1998;21:1259–72. doi: 10.1016/s0896-6273(00)80646-7. [DOI] [PubMed] [Google Scholar]
- Lapetina S, Mader CC, Machida K, Mayer BJ, Koleske AJ. Arg interacts with cortactin to promote adhesion-dependent cell edge protrusion. The Journal of cell biology. 2009;185:503–19. doi: 10.1083/jcb.200809085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YC, Koleske AJ. Mechanisms of synapse and dendrite maintenance and their disruption in psychiatric and neurodegenerative disorders. Annual review of neuroscience. 2010;33:349–78. doi: 10.1146/annurev-neuro-060909-153204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YC, Yeckel MF, Koleske AJ. Abl2/Arg controls dendritic spine and dendrite arbor stability via distinct cytoskeletal control pathways. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:1846–57. doi: 10.1523/JNEUROSCI.4284-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGrath SM, Koleske AJ. Arg/Abl2 modulates the affinity and stoichiometry of binding of cortactin to F-actin. Biochemistry. 2012;51:6644–53. doi: 10.1021/bi300722t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mader CC, Oser M, Magalhaes MA, Bravo-Cordero JJ, Condeelis J, Koleske AJ, et al. An EGFR-Src-Arg-cortactin pathway mediates functional maturation of invadopodia and breast cancer cell invasion. Cancer research. 2011;71:1730–41. doi: 10.1158/0008-5472.CAN-10-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mash DC, ffrench-Mullen J, Adi N, Qin Y, Buck A, Pablo J. Gene expression in human hippocampus from cocaine abusers identifies genes which regulate extracellular matrix remodeling. PloS one. 2007;2:e1187. doi: 10.1371/journal.pone.0001187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matejas V, Hinkes B, Alkandari F, Al-Gazali L, Annexstad E, Aytac MB, et al. Mutations in the human laminin beta2 (LAMB2) gene and the associated phenotypic spectrum. Human mutation. 2010;31:992–1002. doi: 10.1002/humu.21304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AL, Wang Y, Mooseker MS, Koleske AJ. The Abl-related gene (Arg) requires its F-actin-microtubule cross-linking activity to regulate lamellipodial dynamics during fibroblast adhesion. The Journal of cell biology. 2004;165:407–19. doi: 10.1083/jcb.200308055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moresco EM, Donaldson S, Williamson A, Koleske AJ. Integrin-mediated dendrite branch maintenance requires Abelson (Abl) family kinases. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:6105–18. doi: 10.1523/JNEUROSCI.1432-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagar B, Hantschel O, Young MA, Scheffzek K, Veach D, Bornmann W, et al. Structural basis for the autoinhibition of c-Abl tyrosine kinase. Cell. 2003;112:859–71. doi: 10.1016/s0092-8674(03)00194-6. [DOI] [PubMed] [Google Scholar]
- O’Roak BJ, Deriziotis P, Lee C, Vives L, Schwartz JJ, Girirajan S, et al. Exome sequencing in sporadic autism spectrum disorders identifies severe de novo mutations. Nature genetics. 2011;43:585–9. doi: 10.1038/ng.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkstaff JK, Detterich J, Lynch G, Gall C. Integrin subunit gene expression is regionally differentiated in adult brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:1541–56. doi: 10.1523/JNEUROSCI.19-05-01541.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sfakianos MK, Eisman A, Gourley SL, Bradley WD, Scheetz AJ, Settleman J, et al. Inhibition of Rho via Arg and p190RhoGAP in the postnatal mouse hippocampus regulates dendritic spine maturation, synapse and dendrite stability, and behavior. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:10982–92. doi: 10.1523/JNEUROSCI.0793-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanco A, Szekeres C, Patel N, Rao S, Campbell K, Kreidberg JA, et al. Netrin-1-alpha3beta1 integrin interactions regulate the migration of interneurons through the cortical marginal zone. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:7595–600. doi: 10.1073/pnas.0811343106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanis KQ, Veach D, Duewel HS, Bornmann WG, Koleske AJ. Two distinct phosphorylation pathways have additive effects on Abl family kinase activation. Molecular and cellular biology. 2003;23:3884–96. doi: 10.1128/MCB.23.11.3884-3896.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threadgill R, Bobb K, Ghosh A. Regulation of dendritic growth and remodeling by Rho, Rac, and Cdc42. Neuron. 1997;19:625–34. doi: 10.1016/s0896-6273(00)80376-1. [DOI] [PubMed] [Google Scholar]
- van Schijndel JE, van Loo KM, van Zweeden M, Djurovic S, Andreassen OA, Hansen T, et al. Three-cohort targeted gene screening reveals a non-synonymous TRKA polymorphism associated with schizophrenia. Journal of psychiatric research. 2009;43:1195–9. doi: 10.1016/j.jpsychires.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:6810–8. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Miller AL, Mooseker MS, Koleske AJ. The Abl-related gene (Arg) nonreceptor tyrosine kinase uses two F-actin-binding domains to bundle F-actin. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:14865–70. doi: 10.1073/pnas.251249298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren MS, Bradley WD, Gourley SL, Lin YC, Simpson MA, Reichardt LF, et al. Integrin beta1 signals through Arg to regulate postnatal dendritic arborization, synapse density, and behavior. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:2824–34. doi: 10.1523/JNEUROSCI.3942-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver AM, Karginov AV, Kinley AW, Weed SA, Li Y, Parsons JT, et al. Cortactin promotes and stabilizes Arp2/3-induced actin filament network formation. Current biology : CB. 2001;11:370–4. doi: 10.1016/s0960-9822(01)00098-7. [DOI] [PubMed] [Google Scholar]
- Wiggins AT, Pacchioni AM, Kalivas PW. Integrin expression is altered after acute and chronic cocaine. Neuroscience letters. 2009;450:321–3. doi: 10.1016/j.neulet.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Reddy DS. Integrins as receptor targets for neurological disorders. Pharmacology & therapeutics. 2012;134:68–81. doi: 10.1016/j.pharmthera.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]


