Abstract
Malignant degeneration frequently arises from preexisting plexiform neurofibroma in patients with neurofibromatosis type 1 (NF1). Image guided biopsy for diagnostic purposes, such as with CT guidance, can be technically challenging in these patients, as CT cannot distinguish malignant from benign areas within the same tumor. Navigation with multi-modality (PET, CT, and ultrasound) image fusion facilitated the successful biopsy and diagnosis of angiosarcoma arising from a pelvic neurofibroma in a patient with NF1. Successful targeting assisted treatment selection in this case. This novel navigation technique may facilitate the otherwise difficult diagnosis of malignancy in patients with NF1. Pediatr Blood Cancer.
Keywords: FDG-PET, image guided biopsy, malignant peripheral neural sheath tumor, neurofibromatosis type 1, plexiform neurofibroma
INTRODUCTION
NF1 predisposes affected individuals to the development of multiple benign and malignant neoplasms. Malignant degeneration that frequently arises from preexisting histologically benign plexiform neurofibromas may be difficult to diagnose solely based upon clinical symptoms and non-invasive imaging criteria [1]. Early diagnosis, however, is critical, as complete surgical resection is the only curative treatment option. At the same time, tumors arising in NF1 patients may be technically challenging to biopsy using conventional CT-guidance, which may not differentiate between benign and malignant soft tissue. The presence of both benign and malignant areas within the same tumor may lead to sampling error and delayed diagnosis. Recently developed navigation technology and multimodality image fusion enabled successful biopsy of a suspicious mass in a complex NF1 case by targeting the needle to the metabolically active tissue [2]. Successful lesion targeting in this case was critical to treatment selection.
CASE REPORT
An 18-year-old female patient with NF1 presented with a 17-month history of worsening right lower extremity pain, motor and sensory loss, and weight loss. The patient was diagnosed with NF1 at age 2 after presenting with multiple café-au-lait macules and bilateral optic gliomas. At the age of 17 she developed right pelvic pain, and the diagnosis of a right-sided lumbosacral plexiform neurofibroma was made based on MRI. Flourine 18 fluorodeoxyglucose positron emission tomography (18F-FDG-PET) from an outside facility revealed an area of increased uptake of FDG with an SUVmax of 8.2. Worsening pain, foot weakness, and sensory changes over the subsequent 9 months raised concern for malignancy. At this point in time, the patient’s right lower extremity demonstrated diminished strength (2–4/5), absent patellar reflex and complete right foot drop. Magnetic resonance imaging (MRI) of the pelvis demonstrated an 11 × 5 × 8 cm3 ill-defined, heterogeneously enhancing mass arising from the right lumbosacral plexus (Figs. 1a and b). Repeat FDG-PET imaging demonstrated marked FDG uptake within the periphery of this mass (SUVmax = 15.2), and decreased central uptake (Fig. 1). Imaging and clinical findings were suspicious for malignant degeneration of a pre-existing right lumbosacral plexiform neurofibroma (PN) to a malignant peripheral nerve sheath tumor (MPNST). Image guided biopsy was pursued to confirm this diagnosis and facilitate further management [1]. Multimodality image fusion and real-time device tracking were employed to facilitate sampling from the patient’s markedly abnormal foci of FDG uptake.
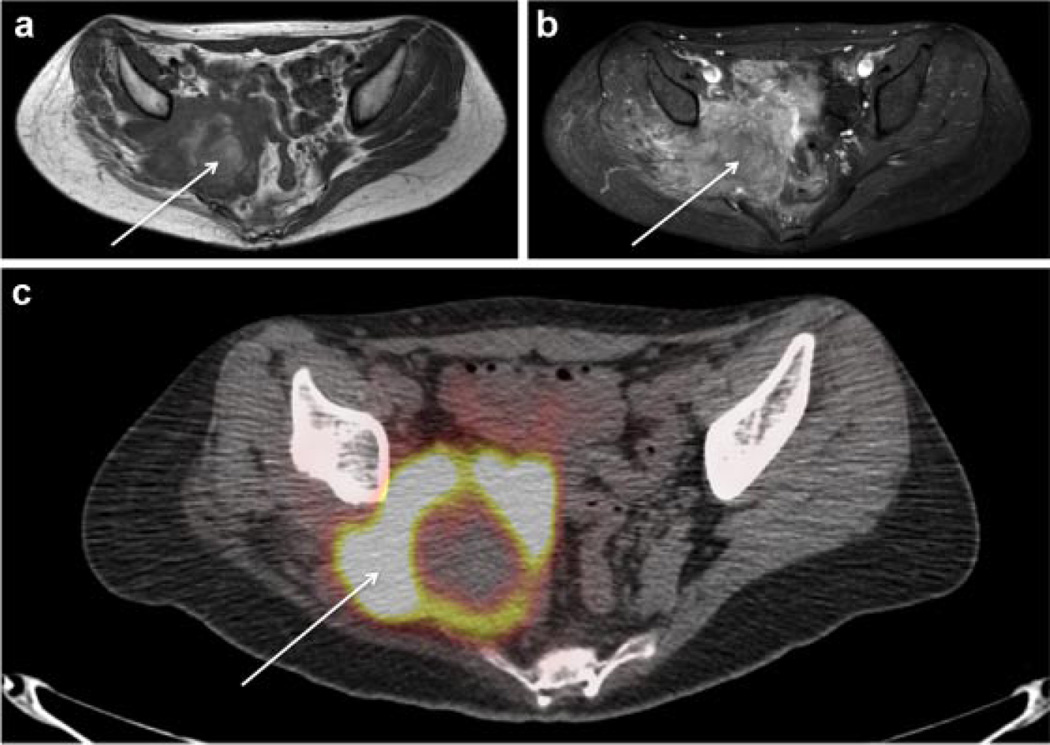
Fig. 1.
MRI and 18F-FDG-PET/CT of right pelvic angiosarcoma in NF1. Axial non-contrast T1-weighted MRI of the pelvis (a) axial post-contrast T1-weighted MRI of the pelvis with fat suppression (b) and 18F-FDG-PET/CT image of the pelvis (c) demonstrate an 11 × 5 × 8 cm3 T1 heterogeneous enhancing pelvic mass (white arrows) with marked peripheral FDG avidity (SUVmax 15.2) of variable thickness, and central photopenia, suggesting central necrosis.
Pre-procedural 18F-FDG-PET/CT (Discovery ST; GE Healthcare, Milwaukee, WI), intra-procedural computed tomography (CT) (Brilliance iCT; Philips Healthcare, Cleveland, OH), and ultrasound (US) datasets (Philips iu22, Philips Healthcare, Cleveland, OH) were co-registered, enabling display of superimposed multimodality imaging for guidance, with real-time updating of the needle position in relation to these pre-procedural images (Fig. 2). Co-registration between US and PET/CT coordinates was accomplished by registering the procedural CT to US via fiducial-based rigid registration and then registering the procedural CT to PET/CT data via manual registration with visual matching of landmarks. The tip of the needle introducer, containing an electromagnetic (EM) sensor coil, was tracked by an EM field generator (Northern Digital, Waterloo, Canada) placed in proximity to the patient. This enabled real-time needle tip localization throughout the procedure, as opposed to only intermittent freeze-frame displays of needle angle and position, as would be the case for conventional CT-guided biopsy. The US transducer was tracked in the same fashion. Needle position was displayed in relation to pre-procedural PET as well as intra-procedural CT and US using custom software (Philips Research, Briarcliff Manor, NY).
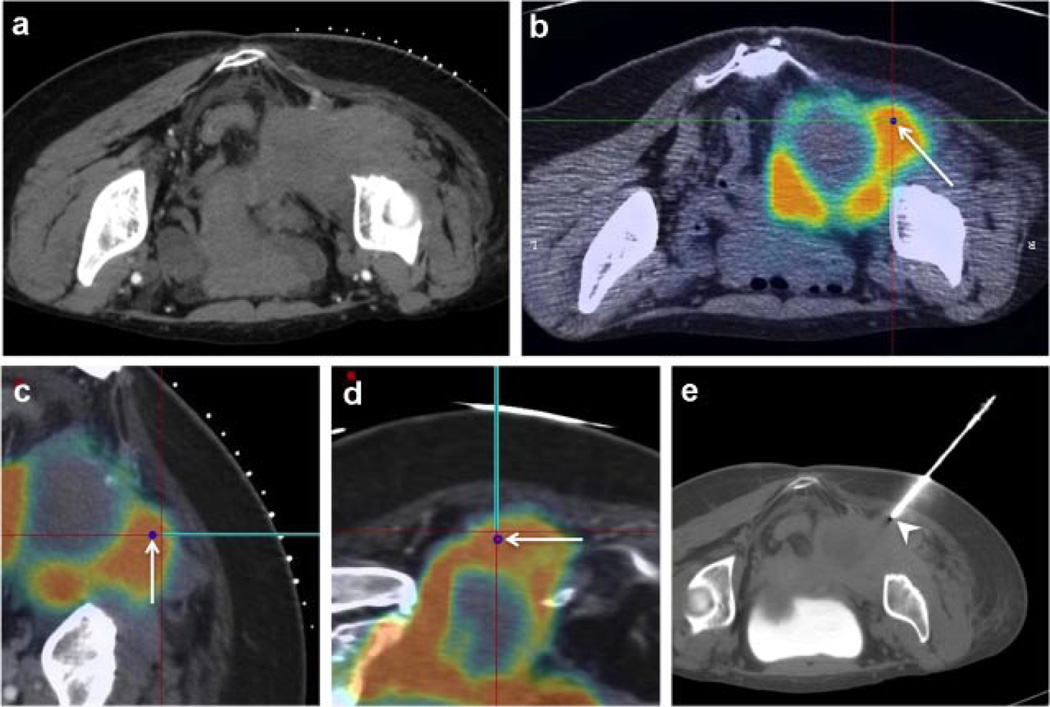
Fig. 2.
Multimodality FDG-PET-guided biopsy with needle navigation diagnoses angiosarcoma within a background of plexiform neurofibroma. (a) Intra-procedural non-contrast CT lacks real-time functional imaging information concerning right pelvic tumor. (b) Image registration enables multiplanar display of intraprocedural CT fused to pre-acquired FDG-PET as well as selection of maximal SUV site as the biopsy target (blue dot highlighted by arrow). (c,d) Electromagnetic device tracking enables real-time display of tracked needle (teal line) over fused intra-procedural CT and pre-acquired FDG-PET throughout the intervention. (e) CT of needle introducer immediately prior to needle biopsy (needle introducer, white arrowhead) confirms sampling location.
Multiple 16-gauge core needle biopsies (Temno Biopsy System; Cardinal Health, Dublin, OH) were obtained from the FDG-avid aspects of the patient’s right pelvic mass (Fig. 2e). Pathology revealed a high grade angiosarcoma arising within a benign PN. Atypical cells stained for CD34 and factor VIII, consistent with endothelial origin. A co-existing MPNST could not be ruled out. As the angiosarcoma was not amenable to complete surgical resection, chemotherapy with paclitaxel was initiated. After a short period of disease stabilization, the patient developed severe hematuria, likely resulting from bladder invasion by the tumor. A course of palliative radiation was administered. The patient was referred to hospice care for palliation of severe pelvic and leg pain. The patient died of disease approximately 4 months after establishing the diagnosis of angiosarcoma.
DISCUSSION
NF1 is characterized by a propensity to develop multiple, anatomically complex tumors, with overlap in clinical symptoms and imaging features between benign and early malignant lesions. Pain, neurological compromise, and rapid tumor enlargement may occur in both MPNST and benign PN [1]. Heterogeneous enhancement after contrast, ill-defined borders and fat-plane invasion on MRI, all present in this case, are associated with, but not exclusive to MPNST [3,4]. High SUVmax, a measure of metabolic activity on 18F-FDG-PET, is observed in the setting of MPNST. However, no specific SUVmax cut-off reliably separates malignant and benign tumors [5,6]. In addition, prior radiotherapy or active inflammation/infection may also elevate FDG avidity in the absence of neoplasm [7]. Lastly, because MPNST is the most common soft tissue sarcoma arising in individuals with NF1 many studies describing radiologic-pathologic correlations for NF1 may not be directly applicable to angiosarcoma [8]. Because accurate diagnosis of malignancy in the setting of NF1 cannot be assured from any set of clinical or radiographic indicators, biopsy is required for diagnosis [1,3].
Conventional image-guided biopsy in the setting of NF1 may be hampered given the complex morphologies and heterogeneous features of NF1 tumors. Frequently, suspicious masses in NF1 arise from numerous, heterogeneous, and partially conjoining growths, within which a single FDG-avid focus may be observed. When foci such as this must be sampled to exclude malignancy, it may be difficult, if not impossible, to delineate the target from adjacent heterogeneous tissue on conventional CT. Such scenarios require the radiologist to rely on his or her spatial memory of the target location on 18F-FDG-PET and estimate the same location on CT [2]. When conventional image-guided biopsy technique fails to accurately sample a target, there is the potential for delayed diagnosis and treatment.
Multimodality image-fusion and real-time device tracking may overcome these challenges. This recently developed technology involves image fusion between pre-procedural 18F-FDG-PET and intra-procedural CT and US imaging, enabling display of focal, FDG-avid targets to the operator throughout the intervention. Electromagnetic device tracking enables tracking of the biopsy needle and US, with display of real-time needle position on multimodality fused images. This has the potential for improved accuracy and reduced unintended collateral damage compared to conventional technique [2]. In this case, FDG-avid sites were safely and exclusively sampled from this complex, heterogeneous pelvic tumor and an unexpected diagnosis of angiosarcoma was made.
Previous case reports have described the development of angiosarcoma in the setting of NF1, and also MPNST with focal divergent differentiation to angiosarcoma, although these entities are far more rare than the more common MPNST [9]. In the case presented only angiosarcoma was identified, but co-existing MPNST could not be ruled out. On the basis of the biopsy results, chemotherapy with paclitaxel was initiated (rather than an ifosfamide and doxorubicin-based regimen for MPNST). Multimodality fusion and needle navigation enable real-time 18F-FDG-PET guided biopsy in NF1. The combination of multimodality image-guidance and real-time needle navigation for percutaneous biopsy may be of value for similar patients with NF1, or for patients with heterogeneity within the tumor.
ACKNOWLEDGEMENTS
This research was supported in part by the Intramural Research program of the NIH, by the NIH Clinical Center and by the National Cancer Institute.
Y.K.: The year long research fellowship for Y.K. was made possible through the National Institutes of Health (NIH) Medical Research Scholars Program, a public–private partnership by the NIH and the generous contributions to the Foundation for the NIH from Pfizer Inc, The Leona M. and Harry B. Helmsley Charitable Trust, and the Howard Hughes Medical Institute, as well as other private donors. For a complete list, please visit the Foundation website at (http://www.fnih.org/work/programs-development/medical-research-scholars-program). The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. B.J.W. and A.M.V.: Research was supported by the NIH Intramural Research Program, the NIH Center for Interventional Oncology, and through a Cooperative Research and Development Agreement (CRADA) between the NIH Center for Interventional Oncology and Philips Healthcare (Best, Netherlands). NIH may have intellectual property in the area. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
CONFLICT OF INTEREST
B.C.W. and F.H.: None.
REFERENCES
- 1.Widemann BC. Current status of spofradic and neurofibromatosis type 1-associated malignant peripheral nerve sheath tumors. Curr Oncol Rep. 2009;11:322–328. doi: 10.1007/s11912-009-0045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Venkatesan AM, Kadoury S, Abi-Jaoudeh N, et al. Real-time FDG PET guidance during biopsies and radiofrequency ablation using multimodality fusion with electromagnetic navigation. Radiology. 2011;260:848–856. doi: 10.1148/radiol.11101985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abreu E, Aubert S, Wavreille G, et al. Peripheral tumor and tumor-like neurogenic lesions. Eur J Radiol. 2013;82:38–50. doi: 10.1016/j.ejrad.2011.04.036. [DOI] [PubMed] [Google Scholar]
- 4.Herendael BH, Heyman SRG, Vanhoenacker FM, et al. The value of magnetic resonance imaging in the differentiation between malignant peripheral nerve-sheath tumors and non-neurogenic malignant soft-tissue tumors. Skeletal Radiol. 2006;35:745–753. doi: 10.1007/s00256-006-0160-y. [DOI] [PubMed] [Google Scholar]
- 5.Meany H, Dombi E, Reynolds J, et al. 18-fluorodeoxyglucose-positron emission tomography (FDG-PET) evaluation of nodular lesions in patients with Neurofibromatosis type 1 and plexiform neurofibromas (PN) or malignant peripheral nerve sheath tumors (MPNST) Pediatr Blood Cancer. 2013;60:59–64. doi: 10.1002/pbc.24212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferner RE, Golding JF, Smith M, et al. [18F]2-fluoro-2-deoxy-d-glucose positron emission tomography (FDG PET) as a diagnostic tool for neurofibromatosis 1 (NF1) associated malignant peripheral nerve sheath tumours (MPNSTs): A long-term clinical study. Ann Oncol. 2008;19:390–394. doi: 10.1093/annonc/mdm450. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi K, Bhargava P, Raja S, et al. Image-guided biopsy: What the interventional radiologist needs to know about PET/CT. Radiographics. 2012;32:1483–1501. doi: 10.1148/rg.325115159. [DOI] [PubMed] [Google Scholar]
- 8.Meany H, Widemann B, Ratner N. Malignant peripheral nerve sheath tumors: Prognostic and diagnostic markers and therapeutic targets. In: Upadhyaya M, Cooper DN, editors. Neurofibromatosis Type 1. Berlin, Heidelberg: Springer; 2012. pp. 445–467. [Google Scholar]
- 9.Ducatman BS, Scheithauer BW. Malignant peripheral nerve sheath tumors with divergent differentiation. Cancer. 1984;54:1049–1057. doi: 10.1002/1097-0142(19840915)54:6<1049::aid-cncr2820540620>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]