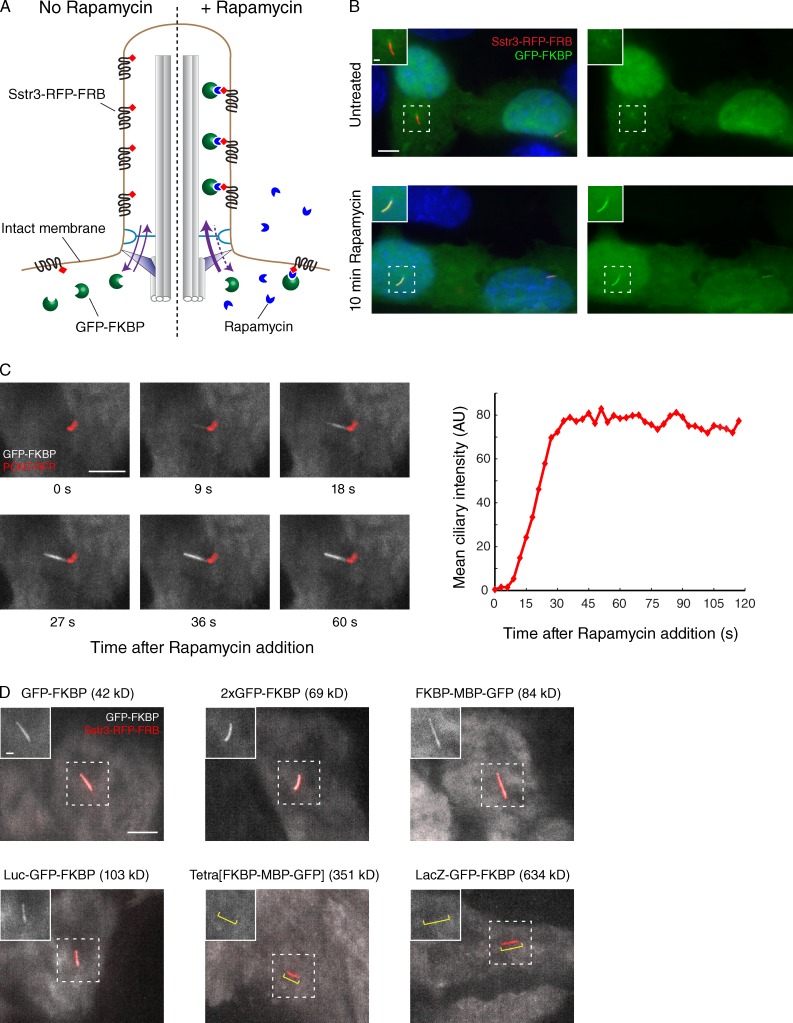
Figure 4.
Inducible diffusion to capture in live cells confirms the existence of a ciliary diffusion barrier. (A) Schematic of the in vivo diffusion to capture assay based on rapamycin-inducible binding of FRB and FKBP domains. Cells expressing Sstr3-RFP-FRB in cilia were transfected with plasmids encoding FKBP- and GFP-bearing fusion proteins. In the absence of rapamycin (left), GFP-FKBP fusions are found in the cytoplasm; after rapamycin addition (right), irreversible dimerization of FRB with FKBP leads to the capture of any GFP-FKBP that diffuses into cilia. (B) Cells were fixed and imaged before and after rapamycin-induced accumulation of GFP-FKBP inside cilia. Insets show enlarged views of cilia. A weak enrichment of GFP-FKBP around the base of cilia is caused by a nonspecific affinity of GFP for pericentriolar material (Fig. S4 B). (C) Pericentrin (PCNT) was cotransfected with GFP-FKBP to mark the base of cilia, and time-lapse imaging was performed after rapamycin addition. Micrographs at select time points are shown on the left, and the integrated intensity of ciliary GFP for the same cell is plotted on the right. 8/8 cells analyzed showed progressive entry of GFK-FKBP from base to tip. AU, arbitrary unit. (D) Fusions of GFP-FKBP with proteins of increasing size reveal the existence of a permeability barrier in live cells. All images were captured by live microscopy 6 min after rapamycin addition. Insets show enlarged views of cilia, and the yellow brackets in the last two images indicate the position of the cilium. Tetra[FKBP-MBP-GFP] denotes FKBP-MBP-GFP fused to a tetramerizing version of the Gcn4 coiled coil (Harbury et al., 1993). Bars: (main images) 5 µm; (insets) 1 µm.