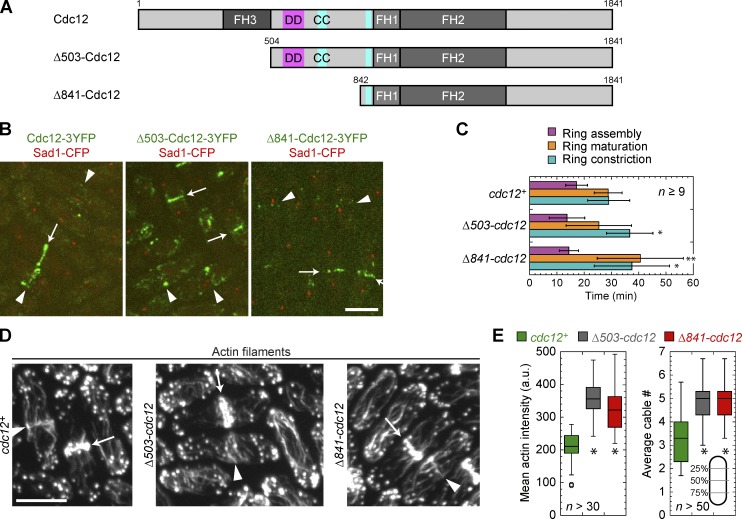
Figure 3.
N-terminal truncations of Cdc12 affect actin cytoskeleton organization. (A) Domain architecture of full-length Cdc12 and two truncations, approximately to scale. Δ503-Cdc12 and Δ841-Cdc12 delete the first 503 aa and the first 841 aa, respectively. FH domains 1, 2, and 3 are in darker shades of gray. Putative dimerization domain (DD, pink) and coiled-coil domains (CC, teal) are shown (Yonetani et al., 2008). (B) Localization of N-terminal truncations of Cdc12 (green) and SPB protein Sad1 (red) as a cell cycle marker. Localizations to ring precursors (arrowheads) and rings (arrows) are shown. (C) Rlc1-3GFP ring assembly, maturation, and constriction (mean ± SD) in Cdc12 N-terminal truncations and wt. *, P < 0.05; **, P < 0.005. (D and E) Staining of actin filaments in wt, Δ503-cdc12, and Δ841-cdc12 cells to reveal an actin meshwork at the division site (arrowheads), actin rings (arrows), and more and brighter actin cables in the mutants. (E, left) Box plots of cell size–corrected integrated actin intensity from the sum intensity projection of 0.4-µm-spaced sections. (right) Box plots of actin cable numbers per cross section in interphase cells. Cables were counted in three line scans of the maximum intensity projection at the positions indicated (gray lines) and the mean number from those three positions for each cell is plotted. *, P < 0.0001 compared with wt. Bars, 5 µm.