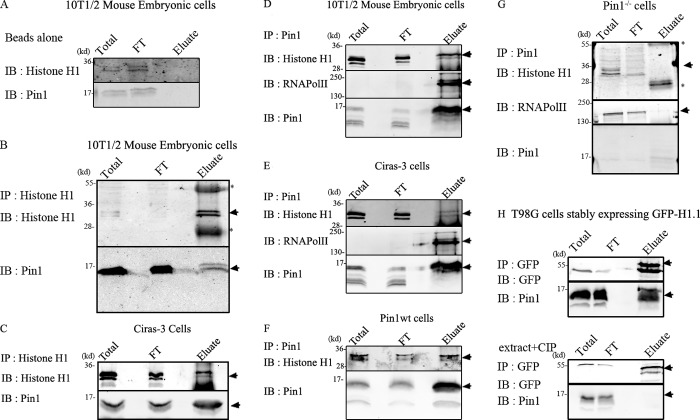
Figure 1.
Pin1 interacts with histone H1. Coimmunoprecipitation experiments were performed to test whether Pin1 and H1 interacted with each other in vivo. “Total” refers to the total nuclear extract before the addition of the antibody, and “FT” refers to flow-through (∼3–6% of the total volume). The entire contents of the eluate were run on the gel. Black lines indicate that intervening lanes were spliced out, and arrows indicate bands that correspond to the protein being IB. Asterisks indicate heavy/light chain IgG antibodies that form part of the eluate. (A) Under the conditions used, both histone H1 and Pin1 did not bind beads nonspecifically. Histone H1 antibodies were used to immunoprecipitate (IP) H1 from mouse embryonic cells (B) and from Ciras-3 cells (C). Immunoblots (IB) reveal pull-down of Pin1 along with histone H1 demonstrating their association in vitro. Reciprocal experiments were performed using Pin1 antibody to pull down Pin1 from extracts prepared from 10T1/2 mouse embryonic cells (D), Ciras-3 cells (E), and Pin1wt cells (F) and Pin1−/− cells (G). RNA polymerase II, which is an established substrate for Pin1, was used as a positive control. Both H1 and RNA polymerase II form a part of the eluate in 10T1/2 and Ciras-3 cells, but not in Pin1−/− cells, demonstrating specific interactions mediated by Pin1. (H, top) Interaction between Pin1 and GFP-H1.1 in extracts prepared from T98G cells stably expressing GFP-H1.1. GFP-H1.1 was immunoprecipitated using GFP antibody coupled to magnetic particles (GFP-Trap). This interaction is dependent on the phosphorylation status of proteins (H, bottom) as treatment of the extracts with calf intestinal phosphatase (CIP), a general nonspecific protein phosphatase, abrogated the interaction between H1 and Pin1.