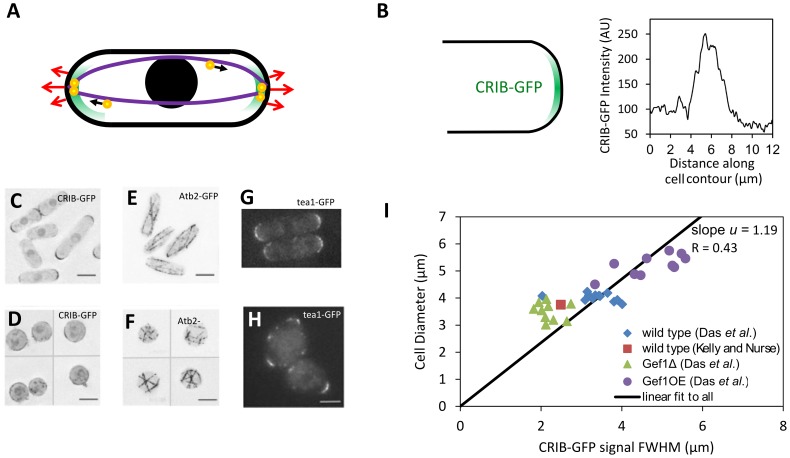
Figure 1. Fission yeast cell shape and regulation by the Cdc42 and microtubule systems.
A. Schematic of fission yeast. Cell outline and nucleus in black, red arrows indicate outward cell wall expansion during bipolar growth, green represents growth-factor Cdc42 signal, purple shows microtubules aligned to the long axis, orange-and-yellow circles are protein-carrying vesicles delivered along microtubules that mark the tips. B. CRIB-GFP (a marker for active Cdc42 [29]) localizes at cell tips. Plot shows CRIB-GFP intensity measured along the contour of a cell tip in Fig. 1A of [15]. C. CRIB-GFP fluorescence in control cells. D. CRIB-GFP after enzymatic digestion of the cell wall that causes cell rounding. CRIB-GFP appears to accumulate in patches along the cell surface. E. Atb2-GFP fluorescence shows microtubules in control cells. In elongated cells microtubules align along the long axis of the cell. F. Atb2-GFP in rounded cells (after enzymatic digestion of the cell wall as in D) shows microtubules with random orientations. G. Tea1-GFP, delivered to cell tips by microtubules shows tip-marker location in wild type cells. H. Tea1-GFP fluorescence in nearly-round sla2Δ cells reveals misplaced tip markers. (C–F: reproduced from [16]; G, H: reproduced with permission from the Journal of Cell Science [57]). I. Cell diameter versus CRIB-GFP signal full-width half-max (measured as in 1B) for wild-type cells and cells with modulated levels of Gef1, a Cdc42 activator. The fit is constrained to go through the origin in order to match the form of the model that predicts the ratio of cell diameter to FWHM. A fit not constrained through the origin gives slope = .57, and intercept 2.15 µm (R = 0.86).