Abstract
BACKGROUND
Actinic keratosis is a frequent lesion which occurs in sunlight exposed areas. Diclofenac sodium and 5-Fluorouracil are effective, non-invasive and easy-to-apply topical treatment options.
OBJECTIVES
To assess and compare the effectiveness of 3% diclofenac sodium associated with 2.5% hyaluronic acid and of 5% 5-Fluorouracil for the treatment of actinic keratosis, as well as the patient's degree of satisfaction and tolerability.
METHODS
28 patients with a clinical diagnosis of actinic keratosis were randomized to receive diclofenac sodium or 5-Fluorouracil and were clinically assessed before and after treatment as well as 8 weeks after the end of treatment. Modified versions of the Investigator and Patient Global Improvement Scores were used.
RESULTS
The average number of lesions in the diclofenac sodium group before and after treatment was 13.6 and 6.6 (p<0,001), respectively, while it was 17.4 and 3.15 (p<0.001) in the 5-Fluorouracil group. There was a significant reduction in the number of lesions in the 5-Fluorouracil group in relation to the diclofenac sodium group (p<0.001). To the non-blinded physician, there was a higher satisfactory therapeutic response in the 5-Fluorouracil group (p<0.001); to the blinded physician, there was a higher satisfactory response in this same group, although not statistically significant (p=0.09). There was a high degree of satisfaction in both groups (73% in the diclofenac sodium group and 77% in the 5-Fluorouracil group; p=0.827). Regarding adverse effects, the diclofenac sodium group presented a higher degree of satisfaction (93.3% vs 38.4%; p=0.008). Erythema, edema, crusts and itching were significantly higher in the 5-Fluorouracil group.
CONCLUSION
We concluded that 5-Fluorouracil was more effective; however, it showed lower tolerability than diclofenac sodium.
Keywords: Diclofenac; Fluorouracil; Keratosis, actinic; Therapeutics
Abstract
FUNDAMENTOS
Ceratose actínica é uma lesão frequente que ocorre em áreas de exposição solar. Diclofenaco sódico e 5-Fluorouracil são opções de tratamento tópico efetivo, não invasivo e de fácil aplicação.
OBJETIVOS
Avaliar e comparar a efetividade do diclofenaco sódico 3% associado ao ácido hialurônico 2,5% e do 5-fluorouracil 5% no tratamento de ceratose actínica, assim como a tolerabilidade e o grau de satisfação do paciente.
MÉTODOS
28 pacientes com diagnóstico clínico de ceratoses actínicas foram randomizados para receber diclofenaco sódico ou 5-fluorouracil e foram avaliados clinicamente antes, ao término e após 8 semanas do tratamento. Utilizou-se o Escore de Melhora Global do Investigador e do Paciente, ambos modificados.
RESULTADOS
A média de lesões no grupo do diclofenaco sódico antes e depois do tratamento foi de 13,6 e 6,6 (p<0,001) e no grupo do 5-fluorouracil foi de 17,4 e 3,15 (p<0,001). Houve uma diminuição significativa no número de lesões no grupo do 5-fluorouracil em relação ao grupo do diclofenaco sódico (p<0,001). Para o médico não cegado houve uma resposta terapêutica satisfatoriamente maior no grupo do 5-fluorouracil (p<0,001); para o cegado, houve uma maior resposta nesse mesmo grupo, porém não significativa (p=0,09). Houve alta satisfação com o tratamento em ambos os grupos (73% no diclofenaco sódico e 77% no 5-fluorouracil; p=0,827). Já em relação aos efeitos adversos, o grupo do diclofenaco sódico apresentou taxa de satisfação maior (93,3% vs 38,4%; p=0,008). Eritema, edema, crostas e prurido foram significativamente maiores no tratamento com 5-fluorouracil.
CONCLUSÕES
Concluímos que o 5-fluorouracil foi mais efetivo, porém apresentou menor tolerabilidade que o diclofenaco sódico.
INTRODUCTION
Actinic keratosis (AK) are hyperplastic epidermal lesions that represent the initial clinical stage of a biological continuum that can culminate in squamous cell carcinoma (SCC).1,2 They usually result from prolonged exposure to non-ionizing radiation, especially from ultraviolet rays of the sun.2
These lesions are very common, being the third main reason for consulting a dermatologist.2 In Brazil, according to the latest census carried out by the Brazilian Society of Dermatology, in 2006, it is the fourth reason for visiting a dermatologist, representing 5.1% of visits.3 Between 1990 and 1999, AK was diagnosed in over 47 million patients in the U.S. With more than one million new cases reported each year, it represents approximately 14% of the visits to the dermatologist.4 In Australia, the estimated prevalence of AK is between 40 and 50% of the population over 40 years old. Other studies in the USA and Australia have shown a lower prevalence, between 11 and 26%.2 In terms of incidence, a study showed that 60% of the people aged 40 or older with a history of AK developed new lesions in 12 months, while only 19% of the people who had no actinic keratosis at the beginning of the study showed new lesions after 12 months.2
Besides its epidemiological impact, AK is also a marker of photodamage, identifying risk groups not only for the development of squamous cell carcinoma, but also for basal cell carcinoma and even melanoma.2 It is the main risk factor for squamous cell carcinoma.2 The probability that actinic keratosis (AK) will evolve into squamous cell carcinoma (SCC) was estimated at 0.075-0.096% per lesion per year.5 Thus, for a person with an average of 7.7 lesions of actinic keratosis on the skin, the incidence of squamous cell carcinoma would be 10.2% in 10 years.6 In 1995, Hurwitz and Monger reported that 97% of cases of SCC are associated with previous actinic keratosis.7 However, they may also regress spontaneously or remain unchanged; its course is unpredictable.8
The most important risk factors for actinic keratosis are the following: white population (Fitzpatrick skin types I and II), age (generally over 30 years old, depending on skin type), sex (higher occurrence in males), geographic areas with higher solar radiation, lifestyle (cumulative exposure to solar ultraviolet radiation and absence of preventive measures) and immunocompromised patients.1
The typical primary lesion is characterized by a rough erythematous papule covered with a white to yellow scale which can range from millimeters to confluent plaques of several centimeters in diameter.6,9 AK usually appears as multiple lesions.9 These lesions are typically concentrated in areas of higher sun exposure, with 80% of them being located on the head, neck and upper extremities.2 There are other variations in their clinical presentation. They may manifest as hyperkeratotic, pigmented, lichenoid, and atrophic AK as well as actinic cheilitis.6,9 Most lesions are asymptomatic, but some may cause itching or a burning sensation.10
The main changes in histopathology are atypical keratinocytes, hyperkeratosis, parakeratosis, especially covering atypical keratinocytes, lichenoid or perivascular lymphocytes, and solar elastosis in the dermis.9
Actinic keratosis may be treated for aesthetic reasons and for relief of symptoms, when they are present. However, the main reason for treatment is to prevent the occurrence of squamous cell carcinoma.9 Various types of therapy are currently available. Among the destructive options are cryotherapy, curettage, excision, dermabrasion, chemical peeling, laser and photodynamic therapy.11 Classic topical options are 5-fluorouracil (5-FU), 5% imiquimod and 3% diclofenac sodiumgel.12,13,14 The latter are the treatments of choice for multiple and microscopic actinic keratosis, for they are easy to apply and effective.8 Other topical agents that are under investigation for the treatment of AK are ingenol mebutate, resiquimod , betulinic acid, piroxicam, 5-FU combined with salicylic acid and the green tea epigallocatechin gallate.15
A comparison of these treatments may help dermatologists to choose the best treatment option for their patient. Considering clinical response, cost and incidence of adverse effects, it is important to compare the results of these methods.
Topical 5% 5-FU is a well-established treatment for actinic keratosis and is approved by the Food and Drug Administration (FDA). It inhibits thymidylate synthetase, an enzyme required for DNA synthesis, causing cell death.16 It is usually applied twice daily for a period of 2-4 weeks.8,10,13 The most common adverse effects are irritation, erythema, dryness and a burning sensation in the skin.12 3% diclofenac sodium associated with 2.5% hyaluronic acid also showed to be effective in the treatment of actinic keratosis in randomized, double-blind, gel vehicle-controlled studies.11 Its mechanism of action is not fully understood, being attributed to a decrease in the formation of arachidonic acid metabolites through inhibition of the enzyme cyclooxygenase. Some of these metabolites inhibit apoptosis and immune surveillance and increase angiogenesis and the invasive ability of tumor cells.11,14 It is approved by the FDA, and its use is recommended twice daily for 60 to 90 days.11 The adverse effects described in the literature include itching, dry skin, erythema and rash.13,14
The general objective of this study was to evaluate and compare the effectiveness of the treatment with 3% diclofenac sodium associated with 2.5% hyaluronic acid gel (DFS) and of 5% 5-fluorouracil cream (5-FU) in the treatment of actinic keratosis on the face and/or scalp and/or back of the hands. The specific objectives were to evaluate and compare tolerability and to assess and compare the patient's degree of satisfaction regarding the efficacy and side effects of these treatments.
MATERIALS AND METHODS
We conducted a randomized, parallel-group clinical trial with a sample of patients of both sexes, over 18 years old, with at least 5 actinic keratosis located on the face and/or back of the hands and/or scalp who sought medical care in a Dermatology Clinic from January to July 2011.
The study excluded pregnant or lactating women, patients on immunosuppressive, immunomodulatory, cytotoxic, retinoid and systemic steroid drugs, patients on some investigational medication or even patients who received treatment for their lesions in the 4 weeks preceding the study. It also excluded volunteers who had other skin diseases in the area to be treated, known sensitivity to any component of the medications under investigation, and patients with less than 5 AK (because topical treatment is indicated for multiple lesions).8
In total, 31 patients were selected and randomized into two groups: Group 1 was instructed to apply 3% diclofenac sodium associated with 2.5% hyaluronic acid gel (formulated at Farmácia de Manipulação B&S, Brazil) twice daily for 12 weeks, and Group 2 was instructed to apply 5% 5-Fluorouracil cream (Efurix; Laboratório Valeant) twice daily for 4 weeks. The length and frequency of the treatment and the vehicles are in accordance with the literature. All patients were instructed to avoid direct sunlight exposure and to use sunscreen.
A non-blinded dermatologist (investigator) evaluated the patients before treatment, reaching the clinical diagnosis of actinic keratosis. The dermatologist determined the exact number of lesions and their location through mapping, marking their location in the drawing contained in the medical evaluation form, and through photographic image (Sony Cyber-Shot 7.2 Mega pixels).
The same dermatologist evaluated the patients immediately after treatment and verified the presence of adverse effects and the patient's degree of pain (rated on a scale of 0 to 10). The dermatologist also evaluated the patients eight weeks after the end of treatment and recorded new photographic images (under the same conditions of light and distance that the first ones were taken) and performed new mapping and counting of the lesions, evaluating improvement of the lesions according to the modified Investigator Global Improvement Score (unchanged, ≤ 50% of the lesions were healed, > 50% of the lesions were healed, all lesions were healed) and the patient's degree of satisfaction through the modified Patient Global Improvement Score (unchanged, < 50% of the lesions were healed, > 50% of the lesions were healed, all lesions were healed), based on the model of the Patient Global Improvement Index.17
A blinded dermatologist evaluated the photographs before and 8 weeks after the end of treatment and rated the improvement of the lesions through the modified Investigator Global Improvement Score, based on the model of the Investigator Global Improvement Index.17 The established criteria for cure are characterized by disappearance of the lesion, presence of mild erythema with no desquamation on the site (it was considered cure with residual erythema) or presence of non-focal, diffuse fine desquamation with no erythema involving the area treated (it was considered cure with presence of xerosis in the region). A satisfactory response was obtained when improvement of the lesions was > 50%, and an unsatisfactory response was obtained when improvement of the lesions was ≤ 50%.
The estimated sample size was 52 volunteers, with 26 cases (A) and 26 controls (B), including 10% for possible losses. We used SPSS (Statistical Package for the Social Sciences) version 15.0 and PEPI (Programs for Epidemiologists) version 3.0 in the data analysis.
In the statistical analysis, we used the t-test for independent and paired (only when it was necessary to compare the results before and after treatment in the same group of volunteers) samples and the chisquare test. The significance level was set at 5% (p <0.05). Some data, after being analyzed, had their results expressed in frequencies, percentages, means and standard deviations.
The project was approved by the Ethics Committee, and the volunteers completed the Informed Consent Form, being notified of the research objectives and having their confidentiality guaranteed during the processes of collecting, storing and analyzing data. All patients were assured that they would receive the necessary care even if unwilling to participate in the study.
RESULTS
Thirty-one (31) patients were initially selected. Three patients in the group treated with 5-FU left the study, one of them due to adverse effects. Thus, 28 patients completed the protocol. The characteristics of the groups are shown in table 1.
TABLE 1.
Baseline characteristics of the patients who completed the treatment
| Diclofenac Sodium | Fluorouracil | P* | ||||
| Patients, No. (%) | 15 (53.6) | 13 (46.4) | ||||
| Age (years) | ||||||
| Average | 74.4 | 71.54 | 0.576 | |||
| SD | ± 8.31 | ± 8.60 | ||||
| Male, No. (%) | 6 (40) | 7 (53.8) | 0.464 | |||
| Fitzpatrick | 0.050 | |||||
| I, No. (%) | 7 ( 46.7) | 4 (30.8) | ||||
| II, No. (%) | 5 (33.3) | 9 (69.2) | ||||
| III, No. (%) | 3 (20) | 0 (0) | ||||
| Treated area | 0.711 | |||||
| Face, No. | 12 | 11 | ||||
| Scalp, No. | 2 | 4 | ||||
| Back of the hands, No. | 3 | 3 | ||||
| Number of lesions | 0.082 | |||||
| Average | 13.6 | 17.4 | ||||
We used the chi-square test to compare the groups.
Of the 28 patients, 15 (53%) had a previous history of skin cancer: 6 in the group treated with DFS and 9 in the group treated with 5-FU (p = 0.122). Regarding family history of skin cancer, 9 patients (32%) had positive history, with no statistically significant difference between the groups (p = 0.339). Considering the number of lesions, most patients (82%) presented more than 10 lesions at the beginning of the treatment.
In the quantitative evaluation of the lesions before and 8 weeks after treatment, we found a statistically significant improvement. In the group treated with DFS, the average number of lesions before treatment was 13.6 (SD ± 4.5) and 6.6 (SD ± 2.94; p <0.001) after treatment. In the group treated with 5FU, the average number of lesions before treatment was 17.4 (SD ± 6.69) and 3.15 (SD ± 2.15; p <0.001) after treatment. Comparing the decrease in the number of lesions in each group, a significant reduction in the number of lesions was observed in the group treated with 5-FU, compared to the group treated with DFS (p <0.001).
When assessing the degree of improvement of the lesions, the blinded dermatologist considered that 66.6% of the patients in the group treated with DFS and 92.3% of the patients in the group treated with 5-FU obtained a satisfactory response (improvement >50%) to treatment (p = 0.09, Table 2).
TABLE 2.
Evaluation of the degree of improvement of the lesions by the blinded dermatologist
| Degree of improvement | Diclofenac Sodium (No.) | 5-Fluorouracil (No.) |
| No improvement | 0 | 0 |
| Improvement < 50% | 5 | 1 |
| Improvement >50% | 10 | 7 |
| 100% of improvement | 0 | 5 |
When assessing response to treatment, the nonblinded dermatologist considered that 33% of the patients in the group treated with DFS and 100% of the patients in the group treated with 5-FU obtained a satisfactory response (p <0.001, Table 3). Kappa test was used to verify inter-observer agreement, which was 0.488, that is, a moderate agreement.
TABLE 3.
Evaluation of the degree of improvement of the lesions by the non-blinded dermatologist
| Degree of improvement | Diclofenac Sodium (No.) | 5-Fluorouracil (No.) |
| No improvement | 0 | 0 |
| Improvement < 50% | 10 | 0 |
| Improvement >50% | 5 | 11 |
| 100% of improvement | 0 | 2 |
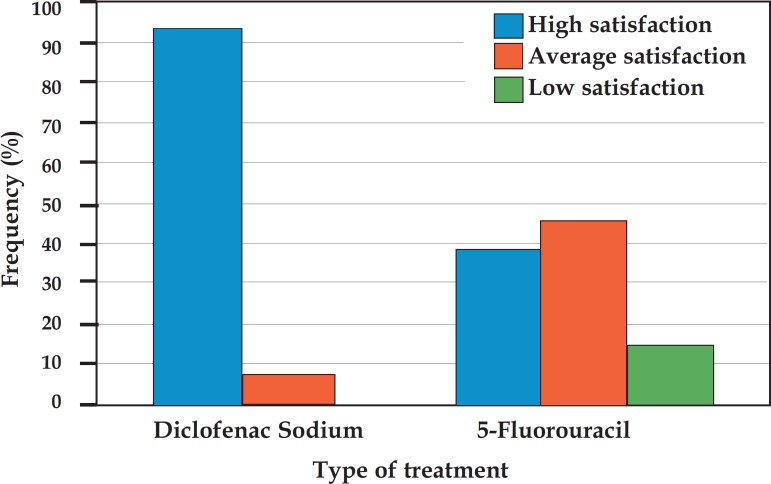
When asked about how satisfied they were with the treatment in terms of improvement of the lesions, most patients reported high satisfaction. In the group treated with DFS, 73% were highly satisfied with the treatment; in the group treated with 5-FU, 77% reported to be highly satisfied. There was no statistically significant difference between the groups (p = 0.827). In relation to satisfaction regarding the adverse effects, the group treated with DFS showed higher satisfaction compared to the group treated with 5-FU, with 93.3% and 38.4% of highly satisfied patients, respectively (p = 0.008 , Graph 1).
GRAPH 1.
Patient satisfaction regarding adverse effects
When asked about the degree of improvement of the lesions, 20% of the patients in the group treated with DFS considered all lesions to be healed compared to 54% of the patients in the group treated with 5-FU (p = 0.05, Graph 2).
GRAPH 2.
Degree of improvement of the lesions, according to the patient
Presence of side effects at the end of treatment and the difference between the groups are shown in table 4. We can observe thats erythema, edema, crusts, itching and discomfort were significantly higher in the group treated with 5-FU. Vesiculation was not found in any patient.
TABLE 4.
Comparison between the groups regarding adverse effects
| Diclofenac Sodium | 5-Fluorouracil | P* | |
| Erythema (%) | 46 | 100 | 0.002 |
| Vesiculation (%) | 0 | 0 | |
| Edema (%) | 0 | 30 | 0.020 |
| Crusts (%) | 33 | 92 | 0.001 |
| Desquamation (%) | 60 | 77 | 0.339 |
| Dry skin (%) | 53 | 46 | 0.705 |
| Discomfort (%) | 13.3 | 53.8 | 0.022 |
| Itching (%) | 13.3 | 53.8 | 0.022 |
| Pain | |||
| Average | 1.21 | 3.60 | 0.300 |
| SD | ± 1.84 | ± 3.61 |
DISCUSSION
Most studies in the literature on the topical treatment of actinic keratosis compare the same treatments at different concentrations or with placebo.8,10,11-14,16,18-20 In 2006, Smith et al. compared the efficacy and tolerability of topical 3% diclofenac sodium and 5% 5-FU, but bilaterally. The two medications obtained similar efficacy, but the 3% diclofenac sodium group presented milder adverse effects.21 In 2007, Krawtchenko et al. conducted a randomized clinical trial comparing 5% 5-Fluorouracil, cryosurgery and 5% imiquimod and showed the superiority of the latter in maintaining cure rates 12 months after treatment as well as in terms of aesthetic results.17 In 2008, Kose et al. compared the use of 3% diclofenac sodium gel with 5% imiquimod cream and found similar effectiveness between the groups, with low rates of complete remission.22 In 2011, Akarsu et al. conducted a study comparing the use of 3% diclofenac sodium and 5% imiquimod and found similar cure rates at the end of treatment in both groups, but with a higher rate of recurrence in the diclofenac sodium group 12 weeks after treatment completion.23
This was a parallel-group study and not a bilateral one and it showed a significant reduction in the number of lesions eight weeks after treatment in both groups, but the group treated with 5-FU showed a greater reduction (an average reduction of 7 lesions in the group treated with DFS and 14.25 lesions in the group treated with 5-FU, p <0.001). It also showed lower tolerability to treatment in the group treated with 5-FU, considering the significantly higher presence of adverse effects in this group at the end of treatment.
According to the literature, treatment with 5-FU should last between 2 and 4 weeks.8,10,13 In this study, patients were instructed to follow the treatment for four weeks. However, the average number of days was 20.3 days, and no patient followed it for less than 14 days. In all cases, the reason why the patients did not comply with the indicated treatment duration was the presence of adverse effects. In the group of volunteers treated with DFS, all patients completed 90 days of treatment, as recommended.
Regarding the evaluation of the medical researchers, the non-blinded dermatologist considered 5-FU the best treatment, since all patients achieved an improvement > 50% in their evaluation and there was a statistically significant difference compared to the group treated with DFS. However, according to the blinded dermatologist's evaluation, there was no statistically significant difference between the treatments, but only a trend toward 5-FU.
It is important to note that the non-blinded dermatologist personally assessed the patients, while the blinded one assessed them only by photographic images. Another interesting fact is that, according to the non-blinded dermatologist, only two patients achieved complete healing of the lesions.
Regarding the patients' evaluation, most were highly satisfied with the improvement of the lesions in both groups, with no statistically significant difference. When considering the degree of improvement, more than half of the patients (54%) in the group treated with 5-FU considered themselves to be fully healed, compared to 20% in the group treated with DFS. The results, however, are different when assessing satisfaction in relation to adverse effects. While 93.3% of the patients in the group treated with DFS are highly satisfied, only 38.4% of the patients in the group treated with 5-FU have the same opinion.
One possible limitation of our study was the lack of a histopathological confirmation of the diagnosis of AK. Furthermore, it is important to note that the results are based on a smaller sample than that calculated as appropriate for the study. Thus, the results could mean a tendency, which could be confirmed if this requirement were met. As the follow-up period of this study was short (eight weeks after completion of treatment), we suggest that further studies with a longer follow-up period be carried out to assess the permanence of the beneficial effects of the treatments.
It is important to mention that some factors observed in this study may characterize information bias, such as the difficulty in evaluating the degree of improvement of the lesions on the part of the blinded physician, who used photographic images only, and the likely embarrassment of some patients in reporting, to the doctor, the adverse effects experienced during the treatment as well as absence of improvement.
CONCLUSIONS
Based on the results of this study, despite its limitations due to its sample size, we conclude that both treatments were effective. However, when comparing the two treatments, 5-FU had a tendency to be more effective.
Tolerability was lower in the group treated with 5-FU.
Patient satisfaction, considering improvement of the lesions, was high in both groups and did not show a statistically significant difference between the two groups. A higher percentage of patients considered their lesions to be healed in the group treated with 5-FU. However, regarding adverse effects, satisfaction was higher in the group treated with DFS, which showed to be the treatment that was better tolerated by the patients.
Footnotes
Financial support: none
Conflict of interests: none
* Work conducted at the Outpatient Clinic of Sanitary Dermatology (Ambulatório de Dermatologia Sanitária - ADS), Health Secretariat of Rio Grande do Sul - Porto Alegre (RS), Brazil.
REFERENCES
- 1.Ramos e Silva M, Castro MCR. Fundamentos de dermatologia. 1 ed. São Paulo: Ateneu; 2009. [Google Scholar]
- 2.Salache SJ. Epidemiology of actinic keratoses and squamous cell carcinoma. J Am Acad Dermatol. 2000;42:4–7. doi: 10.1067/mjd.2000.103342. [DOI] [PubMed] [Google Scholar]
- 3.Sociedade Brasileira de Dermatologia Nosologic profile of dermatologic visits in Brazil. An Bras Dermatol. 2006;81:545–554. [Google Scholar]
- 4.Gupta AK, Cooper EA, Feldman SR, Fleischer AB., Jr A survey of office visits for actinic keratosis as reported by NAMCS, 1990-1999. National Ambulatory Medical Care Survey. Cutis. 2002;70:S8–13. [PubMed] [Google Scholar]
- 5.Marks R, Rennie G, Selwood TS. Malignant transformation of solar keratoses to squamous cell carcinoma. Lancet. 1988;1:795–797. doi: 10.1016/s0140-6736(88)91658-3. [DOI] [PubMed] [Google Scholar]
- 6.Bolognia JL, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. Rio de Janeiro: Elsevier; 2008. [Google Scholar]
- 7.Hurwitz RM, Monger LE. Solar keratosis: an evolving squamous cell carcinoma: Benign or malignant? Dermatol Surg. 1995;21:184–184. doi: 10.1111/j.1524-4725.1995.tb00141.x. [DOI] [PubMed] [Google Scholar]
- 8.Gold MH, Nestor MS. Current treatments of actinic keratosis. J Drugs Dermatol. 2006;5:17–25. [PubMed] [Google Scholar]
- 9.Rapini RP. Dermatopatologia prática. Rio de Janeiro: Di Livros; 2007. [Google Scholar]
- 10.McIntyre WJ, Downs MR, Bedwell SA. Treatment options for actinic keratoses. Am Fam Physician. 2007;76:667–671. [PubMed] [Google Scholar]
- 11.Del Rosso JQ. New and emerging topical approaches for actinic keratoses. Cutis. 2003;72:273-6, 279. [PubMed] [Google Scholar]
- 12.Tutrone WD, Saini R, Caglar S, Weinberg JM, Crespo J. Topical therapy for actinic keratoses, I: 5-fluorouracil and imiquimod . Cutis. 2003;71:365–370. [PubMed] [Google Scholar]
- 13.Stockfleth E, Kerl H, Guideline Subcommittee of the European Dermatology Forum Guidelines for the management of actinic keratoses. Eur J Dermatol. 2006;16:599–606. [PubMed] [Google Scholar]
- 14.Tutrone WD, Saini R, Caglar S, Weinberg JM, Crespo J. Topical therapy for actinic keratoses, II: Diclofenac, colchicine, and retinoids. Cutis. 2003;71:373–379. [PubMed] [Google Scholar]
- 15.Berman B, Cohen DE, Amini S. What is the role of field-directed therapy in the treatment of actinic keratosis? Part 1: overview and investigational topical agents. Cutis. 2012;89:241–250. [PubMed] [Google Scholar]
- 16.de Berker D, McGregor JM, Hughes BR, British Association of Dermatologists Therapy Guidelines and Audit Subcommittee Guidelines for the management of actinic keratoses. Br J Dermatol. 2007;156:222–230. doi: 10.1111/j.1365-2133.2006.07692.x. [DOI] [PubMed] [Google Scholar]
- 17.Krawtchenko N, Roewert-Huber J, Ulrich M, Mann I, Sterry W, Stockfleth E. A randomised study of topical imiquimod vs. topical 5-fluoracil vs. cryosurgery in immunocompetent patients with actinic keratoses: a comparison of clinical and histological outcomes including 1-year follow-up . Br J Dermatol. 2007;157:34–40. doi: 10.1111/j.1365-2133.2007.08271.x. [DOI] [PubMed] [Google Scholar]
- 18.Askew DA, Mickan SM, Soyer HP, Wilkinson D. Effectiveness of 5-fluorouracil treatment for actinic keratosis--a systematic review of randomized controlled trials. Int J Dermatol. 2009;48:453–463. doi: 10.1111/j.1365-4632.2009.04045.x. [DOI] [PubMed] [Google Scholar]
- 19.Gebauer K, Brown P, Varigos G. Topical diclofenac in hyaluronan gel for the treatment of solar keratoses. Australes J Dermatol. 2003;44:40–43. doi: 10.1046/j.1440-0960.2002.00635.x. [DOI] [PubMed] [Google Scholar]
- 20.Fariba I, Ali A, Hossein SA, Atefeh S, Atarzadeh Behbahan SA. Efficacy of 3% diclofenac gel for the treatment of actinic keratoses: A randomized, double-blind, placebo controlled study. Indian J Dermatol Venereol Leprol. 2006;72:346–349. doi: 10.4103/0378-6323.27749. [DOI] [PubMed] [Google Scholar]
- 21.Smith SR, Morhenn VB, Piacquadio DJ. Bilateral comparison of the efficacy and tolerability of 3% diclofenac sodium gel and 5% 5-fluorouracil cream in the treatment of actinic keratoses of the face and scalp. J Drugs Dermatol. 2006;5:156–159. [PubMed] [Google Scholar]
- 22.Kose O, Koc E, Erbil AH, Caliskan E, Kurumlu Z. Comparison of the efficacy and tolerability of 3% diclofenac sodium gel and 5% imiquimod cream in the treatment of actinic keratosis. J Dermatolog Treat. 2008;19:159–163. doi: 10.1080/09546630701818870. [DOI] [PubMed] [Google Scholar]
- 23.Akarsu S, Aktan S, Atahan A, Koç P, Özkan S. Comparison of topical 3% diclofenac sodium gel and 5% imiquimod cream for the treatment of actinic keratoses. Clin Exp Dermatol. 2011;36:479–484. doi: 10.1111/j.1365-2230.2010.03999.x. [DOI] [PubMed] [Google Scholar]