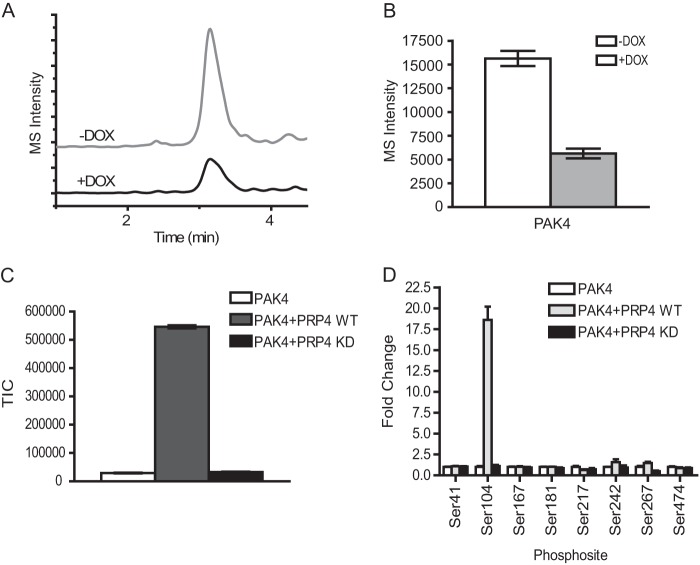
FIGURE 4.
Validation of PRP4 substrates by MRM cell-based assay and in vitro kinase assay. A, extracted ion chromatograms of MRM spectra of transitions (538.5/865.3) monitored for the peptide RDS*PPPPAR of PAK4. An overlay of extracted ion chromatograms of the peptide from control cells without doxycycline treatment (upper panel) and cells with doxycycline (DOX) treatment (lower panel) are shown. B, the Ser104 phosphorylation of PAK4 was decreased by ∼3-fold after induced PRP4 knockdown. Three biological replicates were performed, and the intensities of PAK4 peptide RDS*PPPPAR were averaged and plotted as a bar graph. C, PAK4 kinase can be phosphorylated by recombinant PRP4 in vitro. The in vitro kinase assay was performed as described under “Experimental Procedures.” The resulting reaction mixture was digested by trypsin, and the peptide RDS*PPPPAR from recombinant PAK4 was identified and monitored by Orbitrap. The phosphorylation of the Ser104 residue of PAK4 was significantly increased by PRP4-WT recombinant protein (gray bar) but not by PRP4-KD (black bar). D, survey of all detectable phosphorylated sites in recombinant PAK4 showed that only the phosphorylation of Ser104 residue of PAK4 was significantly up-regulated by PRP4-WT. Standard deviation was calculated and is shown as error bars. TIC, total ion chromatogram.