FIGURE 3.
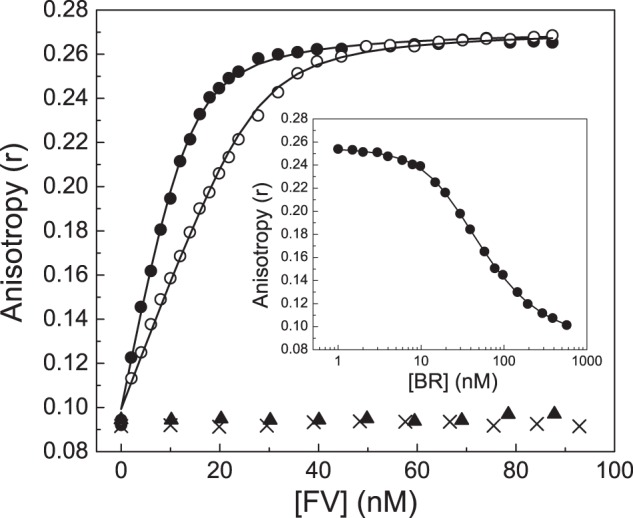
Direct binding of the BR peptide to FV-810. FV-810 was titrated into reaction mixtures containing 20 nm (●) or 40 nm (○) OG488-BR peptide and 50 μm PCPS in assay buffer at 25 °C. Changes in fluorescence anisotropy of OG488-BR were measured as described under “Experimental Procedures.” Lines were drawn after analysis to independent, non-interacting sites with the fitted constants Kd = 2.07 ± 0.2 nm and n = 1.27 ± 0.02 mol of FV-810/mol of OG488-BR at saturation. Control experiments were performed by titrating FV-810 into buffer containing 10 mm EDTA (×) or by titrating rFVa (▴). Inset, the unlabeled BR peptide was titrated into reaction mixtures containing 30 nm OG488-BR, 20 nm FV-810, and 50 μm PCPS. The fitted constants for the unlabeled BR peptide were determined as Kd = 2.1 ± 0.2 nm and n = 1.0 ± 0.06 mol of BR/mol of FV-810 assuming the constants determined above for OG488-BR.
