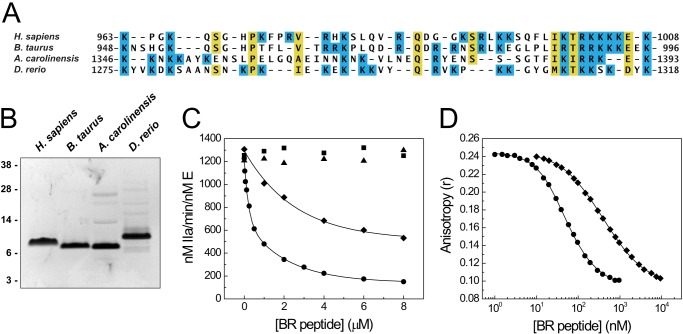
FIGURE 5.
Effect of BR peptides from several species on FV-810 activity. A, multiple sequence alignment of the BR elements from human (Homo sapiens), bovine (B. taurus), lizard (A. carolinensis), and zebrafish (D. rerio) FV proteins. Lysine and arginine residues are shaded in blue. B, purified BR fragments (2 μg each) were resolved by reducing SDS-PAGE and stained with Coomassie Brilliant Blue. C, human (●), bovine (♦), lizard (▴), and zebrafish (■) BR peptides were titrated into reactions containing 1.4 μm prothrombin, 3 μm DAPA, 50 μm PCPS, and 0.1 nm FV-810 in assay buffer at 25 °C. Prothrombin activation was measured as described in the legend to Fig. 1A. D, human (●) and bovine (♦) BR peptides were titrated into reactions containing 30 nm OG488-BR, 20 nm FV-810, and 50 μm PCPS at 25 °C. Fluorescence anisotropy was measured, and equilibrium binding constants were determined assuming a stoichiometry of 1 mol of FV-810/mol of BR peptide: the human BR, Kd = 2.2 ± 0.2 nm; and the bovine BR, Kd = 28.3 ± 0.6 nm.