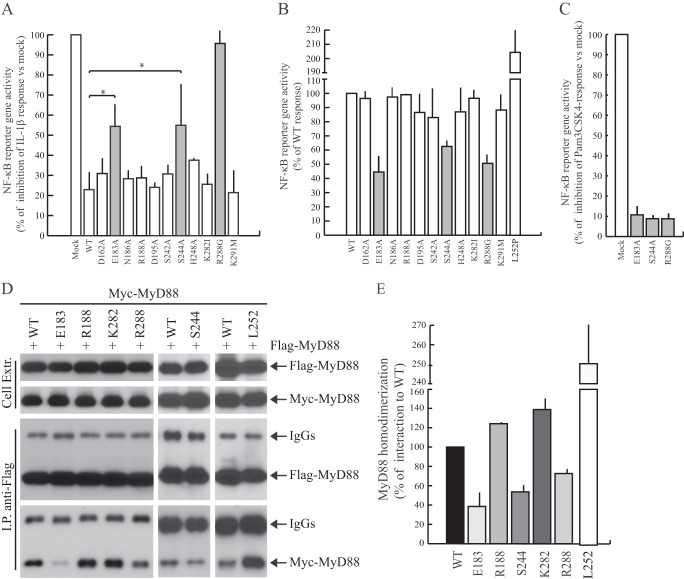
FIGURE 2.
Functional analysis of MyD88 mutants. A, HEK293T cells were transfected with NF-κB reporter construct expressing firefly luciferase alone or in combination with wild-type or mutated Myc-MyD88-TIR. Twenty-four h after transfection, cells were stimulated or not with IL-1β (20 ng/ml) for 6 h and lysed for biocounter luminometer analysis. Data are normalized for transfection efficiency (see “Experimental Procedures”), and each column of the graph indicates the relative luciferase activity of the stimulated cells over the nonstimulated cells. Data are expressed as a percentage of inhibition of IL-1β response ± S.D. (error bars) from three separate experiments. Statistical significance was determined by Student's t test. *, p < 0.05. Gray bars indicate the mutants that exerted a significant increase of NF-κB activity compared with the wild-type protein. B, HEK293T cells were transiently transfected with wild-type (WT) or mutant MyD88 constructs together with NF-κB-luciferase reporter construct. Data were normalized for transfection efficiency as above and expressed as a percentage of wild type ± S.D. from three separate experiments. Gray bars indicate the defective MyD88 mutants. C, THP-1 cells were transfected with NF-κB reporter construct expressing firefly luciferase alone or in combination with wild-type or mutated Myc-MyD88. Twenty-four h after transfection, cells were stimulated or not with Pam3CSK4 (1 μg/ml) for 6 h and lysed for biocounter luminometer analysis. Data are normalized for transfection efficiency as described under “Experimental Procedures,” and each column of graph represents the relative luciferase activity of the stimulated cells over the nonstimulated cells. Data are expressed as a percentage of inhibition of TLR2 response ± S.D. from three separate experiments. D, representative Western blot analysis of the effect of selected mutations on MyD88 homodimerization is shown. HEK293T cells were transfected with FLAG-MyD88 in combination with wild-type (first and sixth lanes) or mutated (second through fifth and seventh lanes) Myc-MyD88. Cell extracts were immunoprecipitated (IP) with anti-FLAG antibody, and the immunoprecipitated proteins were then analyzed with either anti-FLAG or anti-Myc-specific antibodies to detect the interaction. E, densitometric analysis of the effect of MyD88 mutants used in C was performed on MyD88 homodimerization. Data are expressed as a percentage of the wild type ± S.D. from three separate experiments.