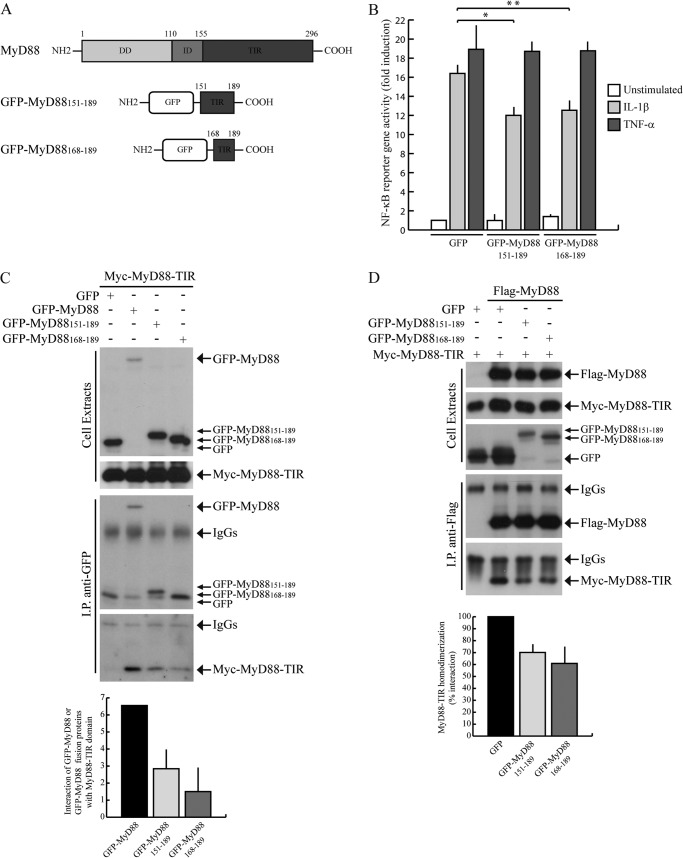
FIGURE 5.
GFP-MyD88151–189 and GFP-MyD88168–189 interfere with IL-1β-induced NF-κB activation. A, schematic representation of GFP-MyD88 mini-proteins. B, NF-κB activity luciferase reporter assay. HEK293T cells were transfected with GFP, GFP-MyD88151–189, or GFP-MyD88168–189 together with NF-κB luciferase reporter construct and stimulated or not with either 20 ng/ml IL-1β (light gray bars) or 20 ng/ml TNF-α (dark gray bars) for 6 h. Data were normalized for transfection efficiency as described in Fig. 2 and expressed as mean -fold induction ± S.D. (error bars), compared with control expressing GFP from three experiments. Statistical significance was determined by Student's t test. *, p < 0.05; **, p < 0.01. C, GFP-MyD88151–189 and GFP-MyD88168–189 interaction with MyD88-TIR domain. HEK293T cells were transfected with Myc-MyD88-TIR in combination with GFP (first lane), GFP-MyD88 (second lane), or GFP-MyD88151–189 and GFP-MyD88168–189 (third and fourth lanes, respectively). Cell extracts were immunoprecipitated (IP) with anti-GFP antibody, and immunoprecipitated proteins were analyzed by Western blotting with either anti-GFP- or anti-Myc-specific antibodies. Densitometric analysis of the degree of interaction of Myc-MyD88-TIR with GFP-MyD88, GFP-MyD88151–189, or GFP-MyD88168–189 is shown in the bar graph. D, GFP-MyD88151–189 and GFP-MyD88168–189 interference with the homodimerization of MyD88-TIR domain. HEK293T cells were transfected with Myc-MyD88-TIR alone (first lane) or in combination with FLAG-MyD88 (second through fourth lanes) in the presence of GFP (first and second lanes), GFP-MyD88151–189 (third lane), or GFP-MyD88168–189 (fourth lane). Cell extracts were immunoprecipitated with anti-FLAG antibody, and immunoprecipitated proteins were analyzed by Western blotting with either anti-FLAG- or anti-Myc-specific antibodies. Densitometric analysis of MyD88-TIR homodimerization in the presence of GFP or GFP-MyD88 fusion proteins is shown and is expressed as percentage of interaction with respect to GFP.