FIGURE 7.
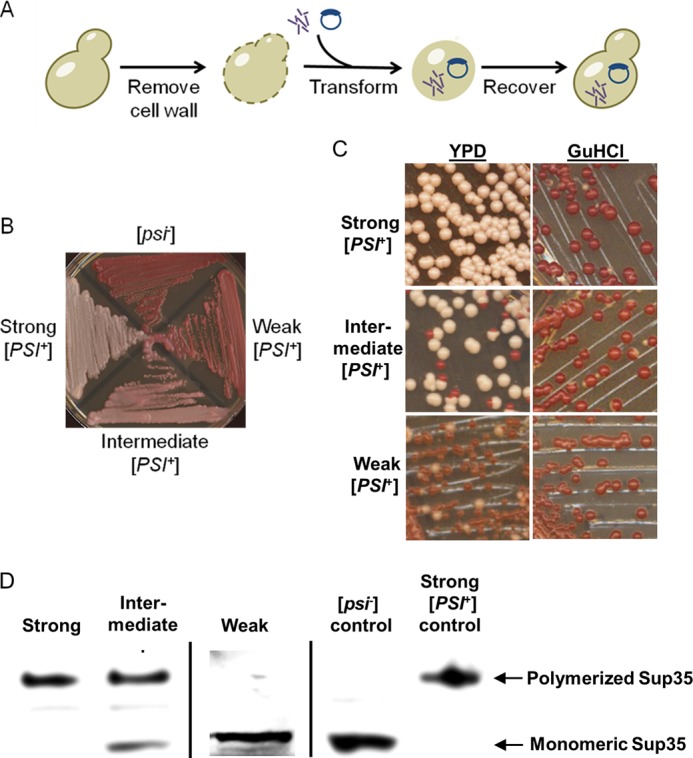
In vivo assay for in vitro generated prion strains. A, transfection experiments were conducted by enzymatically stripping the cell walls of the yeasts and then simultaneously transforming a marker plasmid and amyloid material. The yeast cells were recovered in the stabilizing medium, and resulting colonies were checked for phenotypes. B, [PSI+] strains were classified as strong, intermediate, or weak. C, these [PSI+] strains differed by frequencies of spontaneous mitotic loss of the [PSI+] phenotype, visualized as appearance of red ([psi−]) colonies after three passages on YPD medium, but were all curable after three passages on YPD medium containing 5 mm guanidine hydrochloride, an agent antagonizing prion propagation. D, boiled gel analysis demonstrates that cultures of strong [PSI+] strains contain essentially all Sup35 protein sequestered into an aggregated form (top band), whereas in intermediate strains, some Sup35 protein remains soluble, and in the weak strain, essentially all detectable Sup35 protein is soluble, apparently due to low propagation capabilities of aggregates and gross accumulation of the [psi−] cells. Standard [PSI+] and [psi−] cultures are shown as controls.
