FIGURE 6.
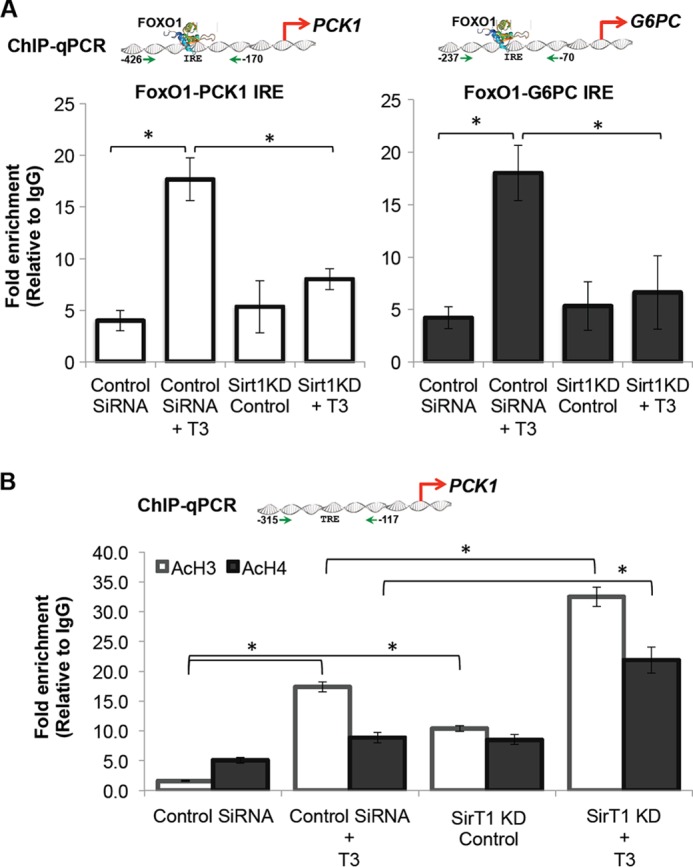
T3 induced FoxO1 recruitment to PCK1 and G6PC promoters in a SirT1-dependent manner. A, ChIP-qPCR analysis revealed that T3 increased FoxO1 recruitment on PCK1 and G6PC promoters at the IRE region (−426 to −170 for PCK1 and −237 to −70 for G6PC gene promoter), which is known for FoxO1 binding in relation to gluconeogenesis. SirT1 deficiency significantly reduced T3-dependent binding of FoxO1. Cells were cultured with control siRNA or SirT1 siRNA for 48 h followed by replacement of medium with normal medium as indicated under “Experimental Procedures.” After 72 h of transfection, cells were treated with T3 for 1 h to analyze FoxO1 recruitment. B, ChIP-qPCR analysis of TRE on PCK1 promoter demonstrates that T3 (0.1 μm) rapidly increased acetylation of histone H3 and H4 in this region. HDAC activity of SirT1 was also confirmed as evident from increased basal acetylation of histone H3 and H4 during SirT1 KD in TRβ-HepG2 cells. During SirT1 KD, T3 (0.1 μm) further increased acetylation of these histones, which is significant. 2 μl of immunoprecipitated DNA (1% input DNA) was used for qPCR analysis as indicated under “Experimental Procedures” (n = 3; *, p < 0.05). Error bars represent mean ± S.D.
