FIGURE 1.
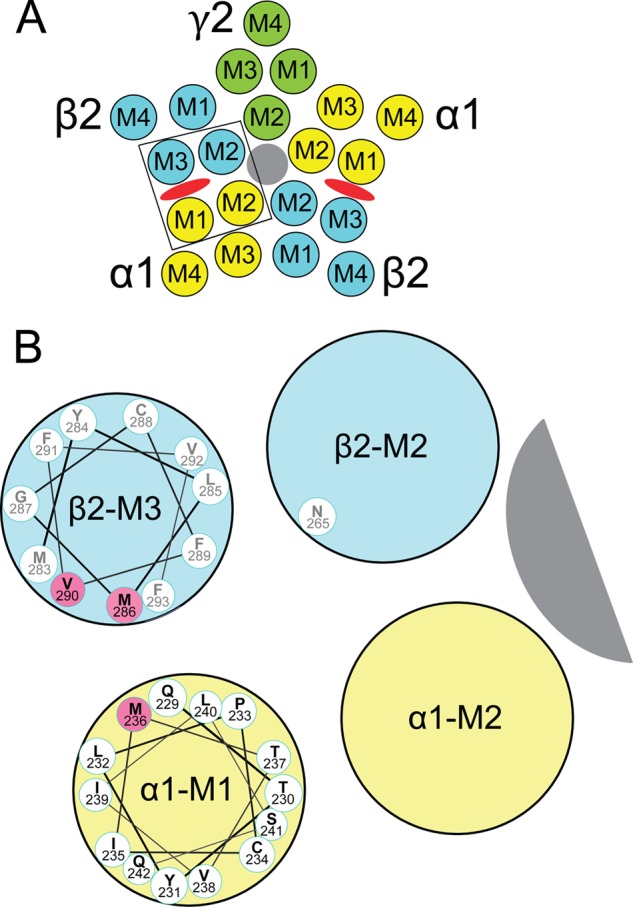
Transmembrane domains and amino acids forming etomidate sites in GABAA receptors. A, a diagram of a α1β2γ2L GABAA receptor cross-sectioned in the membrane plane and viewed from the extracellular space illustrates the arrangement of α1 (yellow), β2 (blue), and γ2 (green) subunits and the transmembrane domains (M1–M4) within each subunit. The chloride channel is shaded gray. Etomidate sites (red ovals) are located between α-M1 and β-M3 domains. B, an expanded diagram of one etomidate site (outlined in A), showing a helical wheel projection of the α1-M1 sequence from Gln-229 (nearest) through Gln-242. The orientation of the side chains is approximate and based on a homology model built from the structure of G. violaceous pentameric ion channels (33). GABAA receptor residues that are photolabeled by etomidate derivatives (13, 14), in both α-M1 and β-M3, are highlighted in pink. We also illustrate β2Asn-265 on β-M2, a residue where mutations influence etomidate sensitivity (37, 38).
