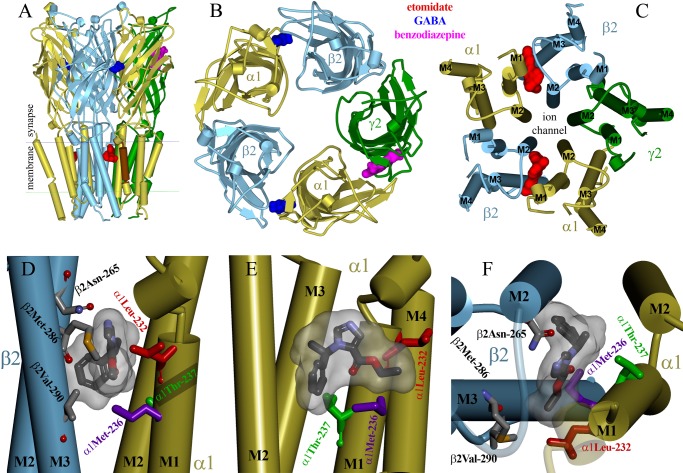
FIGURE 7.
Etomidate docked within an α1β2γ2L GABAA receptor homology model contacts α1Leu-232, α1Met-236, and α1Thr-237 in the M1 domain. A–C, multiple views of a homology model of GABAA receptor built on the structure of pentameric GLIC (Protein Data Bank entry 3P50), showing α-helices as cylinders and β-sheets as ribbons with subunits α1 (yellow), β2 (blue), and γ2L (green). The GABAA receptor-specific ligands GABA (blue), a benzodiazepine (magenta), and etomidate (red) are shown within their intersubunit binding sites as Connolly surfaces. A, the model viewed parallel to the membrane. The extent of the α1-M1 helix examined in this study is colored brown. B, a view from the extracellular space of the extracellular domains. C, a view from the extracellular space of the transmembrane domains. D–F, enlarged views of one etomidate site. Shown in stick format are residues protected by etomidate from pCMBS modification after cysteine substitution, α1Leu-232 (red), α1Met-236 (purple), and α1Thr-237 (green). Also shown in stick format color-coded by atom type (gray, carbon; red, oxygen; blue, nitrogen; gold, sulfur) are residues of interest in the β2 subunit and etomidate docked at its lowest energy orientation. A Connolly surface of the composite of 100 most stable docked poises surrounds the etomidate molecule. D, a view from within the membrane. E, a view from the β2 subunit. F, a view from the synaptic end of the transmembrane domain.