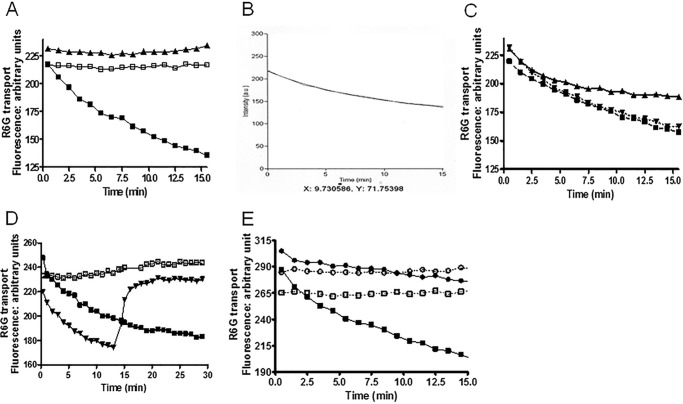
FIGURE 10.
Reduced R6G transport in D1042N PM vesicles demonstrates uncoupling of ATPase activity and transport. We measured quenching of R6G fluorescence in vesicles as described under “Experimental Procedures” using 100 nm R6G and 30 μg of vesicle protein. We show representative plots for each experiment. We performed these with an n value of at least 3, with three independent preparations of purified PM vesicles for each strain. A, dependence of quenching on Pdr5 and ATP in WT vesicles. ▴ = −Pdr5, +3 mm ATP; □, +Pdr5, −ATP; ■, +Pdr5, +3 mm ATP. B, a representative scan of retained fluorescence from WT vesicles in the presence of 3 mm ATP fit to first order kinetics. C, the effect of ATP concentration on the efflux of R6G from WT purified PM vesicles. ▴, 1.5 mm ATP; ▾, 4 mm ATP; ■, 3 mm ATP. D, the effect of adding 1 μm oligomycin at 13 min on quenching. □, +Pdr5, −ATP; ■, +Pdr5, +3 mm ATP; ▾, 3 mm ATP, +Pdr5, +1 μm oligomycin. E, reduced R6G quenching in the D1042N mutant. □, WT minus ATP; ■, WT plus 3 mm ATP; ○, D1042N without ATP; ●, D1042N with 3 mm ATP.