FIGURE 12.
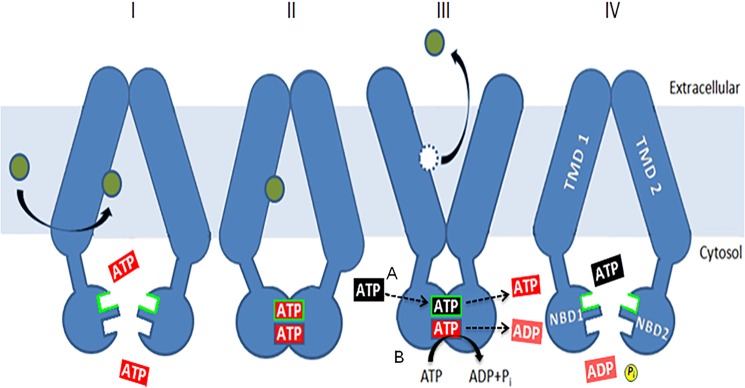
A model for the role of the deviant ATP-binding site. The model is a simple variation on one initially proposed by Gupta et al. (33). ATP (red) is bound at both sites (I), causing dimerization (II) of the NBDs, but hydrolysis is limited to the canonical one. Binding and nucleotide exchange (black for red) at the deviant site (III), indicated by the green border, allow a change from an inward-facing, drug-binding conformation to an outward-facing, drug-releasing conformation. This is mediated by residues such as Glu-1013 and Asp-1042. ATP hydrolysis at the canonical site (IV) restores the transporter to a drug-binding conformation and causes the release of unhydrolyzed ATP (blue). Allosteric inhibition of Pdr5-ATPase activity coming from the transmembrane domains via the intracellular loops works at this step. ATPase activity is not stimulated by drugs, so the cycle is thought to be constitutive in nature. Some of the mutants described here force the nucleotide exchange step to take place along with hydrolysis at the canonical site, as originally proposed by Gupta et al. (33).
