FIGURE 4.
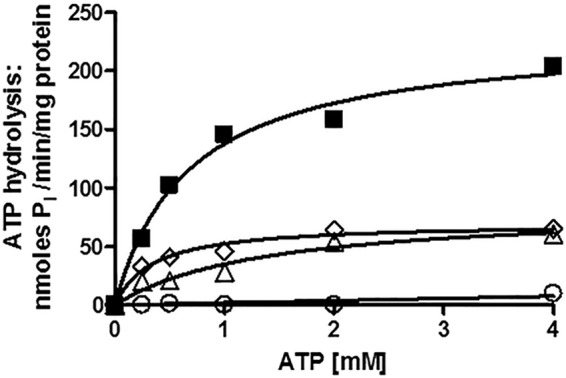
Pdr5-mediated ATPase activity in WT and mutant strains strongly suggests that the deviant ATP site is not a major source of catalytic activity. We measured Pdr5-specific ATPase activity as previously described (20) in double-copy strains with 12 μg of PM vesicle protein in each reaction, carried out for 8 min at 35 °C. Representative plots are shown (n = 3). Activity was assayed in PM vesicles prepared from double-copy strains as initially described (9). We used GraphPad Prism software to create and analyze the plots of activity versus ATP concentration. We subtracted the small (<5%) nonspecific activity observed in PM vesicles prepared from the isogenic ΔPdr5 strain before calculating the final activity. ■, WT; ▵, E1013A; ♢, C199A; ○, G312A.
