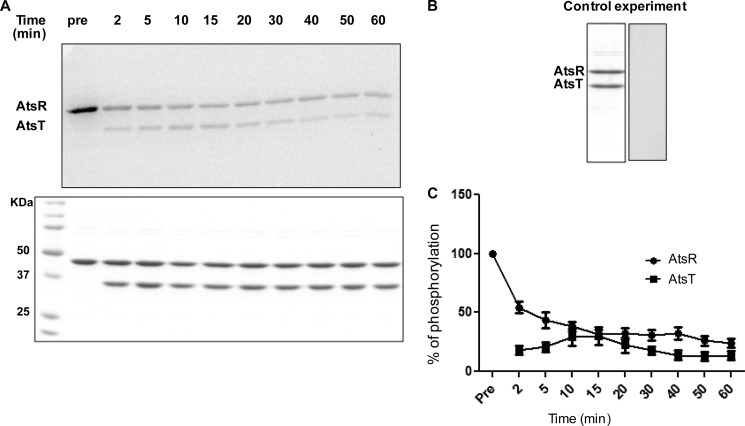
FIGURE 4.
Kinetics of phosphotransfer from AtsR to AtsT. A, 5 μmol of AtsR were preincubated with 5 μCi ([γ-33P]ATP) in a standard phosphorylation mixture (100 mm Tris-HCl, pH 8, 50 mm KCl, 5 mm MgCl2, 1 mm DTT) for 15 min, and then 5 μmol of AtsT and 20 mm ATP were simultaneously added to the reaction. The reaction was chased over time at 25 °C. Aliquots were removed before and after the chase at the times indicated. Reactions were terminated by adding SDS-PAGE loading buffer. Samples were run on 16% SDS-PAGE gel and stained with Coomassie Blue (bottom). Phosphorylated proteins were visualized by a PhosphorImager (top). The images shown here are the representatives of two independent repeats. B, 5 μmol of AtsR was incubated simultaneously with both labeled and unlabeled ATP for 10 min followed by the addition of 5 μmol of AtsT and incubated for 15 min. Samples were run on 16% SDS-PAGE gels and stained with Coomassie Blue (left) or visualized by a PhosphorImager (right). C, the y axis represents the percentage of normalized absorbance of densitometry readings from bands corresponding to phosphorylated proteins obtained from two independent experiments.