FIGURE 2.
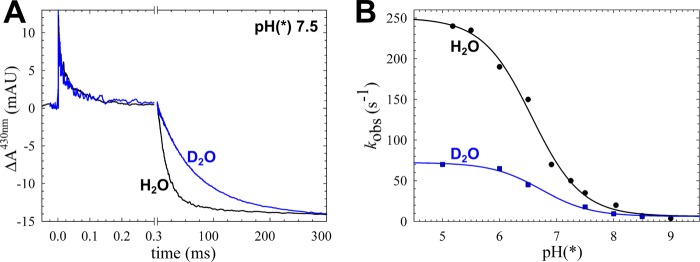
The kinetic (deuterium) isotope effect on ETPT during the reaction between fully reduced cNOR and O2. A, trace obtained at 430 nm of wild type cNOR in H2O or D2O at pH(*) 7.5, showing the change in absorbance (ΔA) over time (with the laser flash set at t = 0). The data were normalized to the COoff step at t = 0 for the rapid time scale and to the amplitude of the ETPT (which varies slightly between experiments) for the longer time scale. The laser artifact at t = 0 has been removed for clarity. The COoff reaction results in a rapid increase of absorbance, and O2 binding (with τ of ∼40–50 μs) then results in a decrease. On the longer time scale, the ETPT is seen as a further negative ΔA (τ of ∼20–25 ms in H2O). B, the rate of the ETPT in D2O (blue) and H2O (black) as a function of pH(*). The D2O data were fitted to a pKa* of 6.7 ± 0.1 and a kH (maximal rate at low pH) of 66 ± 3 s−1, and the H2O data (which are from Ref. 8) were fitted to a pKa of 6.6 ± 0.1 and a kH of 244 ± 7 s−1. mAU, milliabsorbance units.
