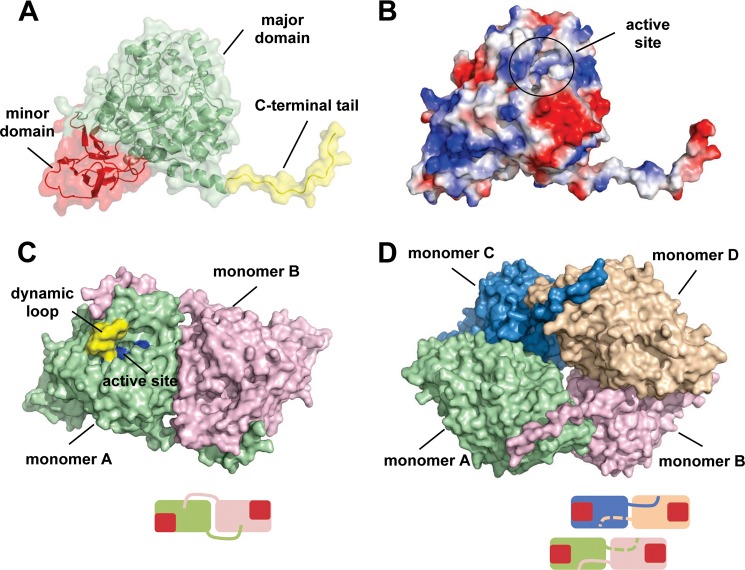
FIGURE 2.
The overall structure of the apo-TnDhp protein. A, monomer TnDhp structure could be divided into a major domain (green), minor domain (red), and C-terminal tail (yellow). B, electrostatic potential surface shows the positive (blue) and negative (red) charge distributions. The active site is located in the major domain with a tunnel for transport of substrates and products. C, the C-terminal tail not only contributes to the main interactions for maintaining the dimeric state of the protein molecules (green, monomer A; pink, monomer B) but also extends to the dynamic loop (yellow) near the active site (blue). D, the tetramer structure is formed by two dimers. The bottom scheme in each panel shows the simplified dimer and tetramer structures. Monomers are shown in different colors, and the red squares represent the locations of the minor domains.