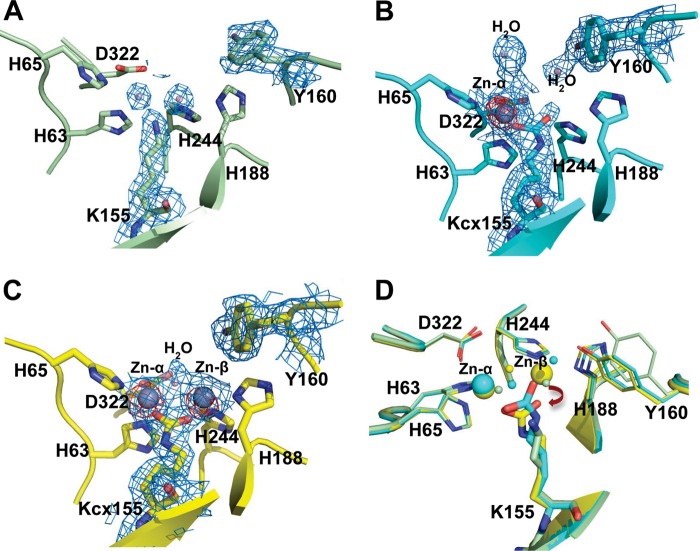
FIGURE 3.
Structures of the active site. The composite omit maps at 1.0 σ are shown in blue mesh, and the zinc anomalous difference maps at 5.0 σ are shown in red mesh. A, the apo-TnDhp contains two waters and a non-carbamylated Lys155 at the active site. B, the mono-Zn TnDhp contains one zinc atom at the Mα site. The electron density clearly indicates that Lys155 is carbamylated. C, two zinc atoms are present in the di-Zn TnDhp. D, structural superimposition of the apo- (light green), mono-Zn (cyan), and di-Zn TnDhp (yellow) shows conformational differences. Displacements of the carbamylated Lys155 in the mono-Zn to the di-Zn TnDhp are highlighted by dark red arrows.