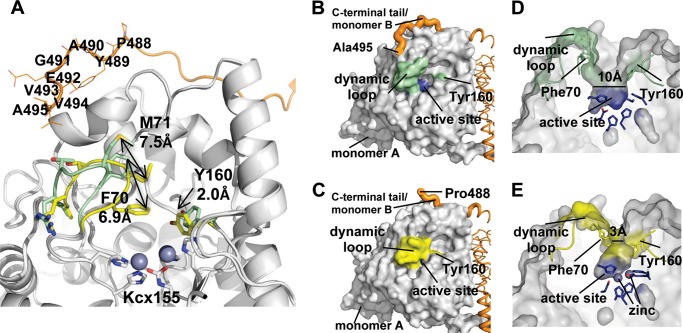
FIGURE 5.
Dynamic loops and the C-terminal tail. A, upon the metal binding, two dynamic loops (Ala69–Arg74 and Met158–Met165) move toward the active site (light green, apo-TnDhp; yellow, di-Zn TnDhp). In particular, Phe70 and Met71 on the loop Ala69–Arg74 swing with maximum distances of 6.9 and 7.5 Å, respectively. The C-terminal tail from another monomer (orange) is shown, and the most dynamic residues are labeled. The carbamylated lysine 155 is labeled as Kcx155. B, the structure of apo-TnDhp shows that the dynamic loops (green) in the “open form” expose the active site (blue) to the molecular surface. The C-terminal tail from another monomer (orange) is shown in close proximity to the dynamic loop. C, the structure of di-Zn TnDhp shows that dynamic loops (yellow) in the “closed form” obstruct the active site. In this state, the C-terminal tail (orange) is too flexible to be modeled, because there is no electron density seen for the last seven residues. D, a side view of the active site with the dynamic loops in the open form. The cross-section bottleneck of the tunnel is about 10 Å (light green), which is wider than the average size of substrates (6 Å). The active site pocket surrounded by key residues is shown in blue. E, a side view of the active site with the dynamic loops in the closed form. The cross-section bottleneck is reduced dramatically to ∼3 Å (yellow). The active site pocket is shown in blue. The two zinc ions are depicted as spheres.