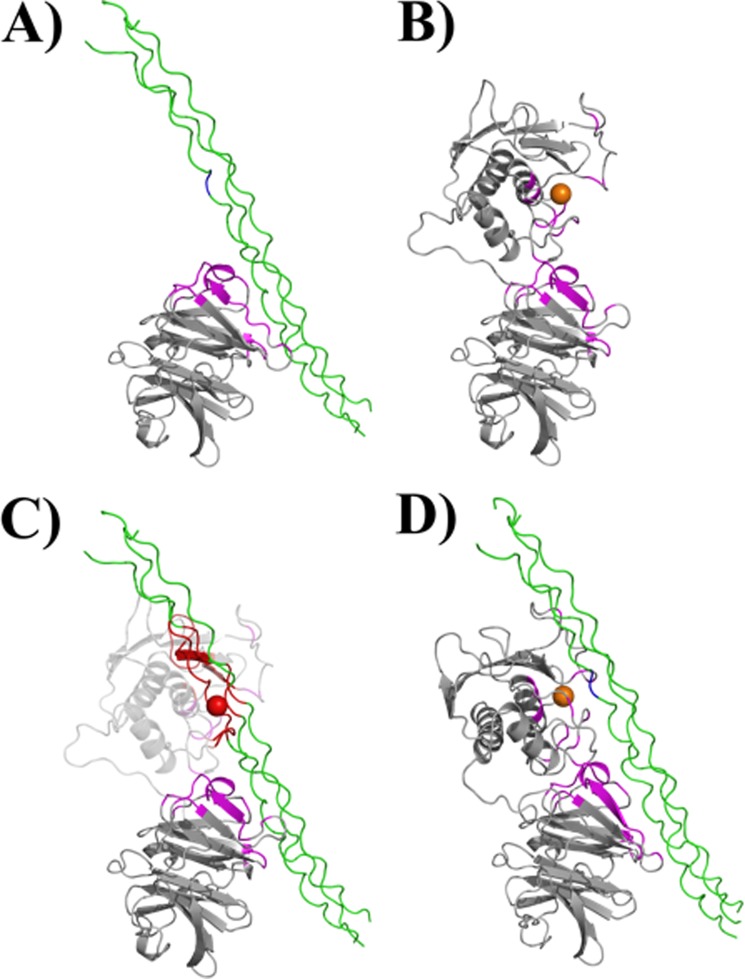
FIGURE 1.
Interaction of MMP-1 with the collagen triple-helix. A, the HPX domain of MMP-1 binds the collagen triple-helix through specific residues in blades I and II (highlighted in magenta; the Gly-Ile cleavage site within the triple-helical peptide is shown in blue). B, experimentally determined regions of CAT and HPX domains involved in binding of the triple-helix are highlighted in magenta. The conformation of MMP-1 is based on the x-ray crystallographic structure 2CLT (active, full-length MMP-1). C, if the 2CLT structure is maintained, binding of the HPX domain to the triple-helix results in the collision of the CAT domain with the triple-helix (residues highlighted in red). D, interdomain flexibility is required for the MMP-1 to correctly approach the substrate, as described for the first step of collagenolysis (9).